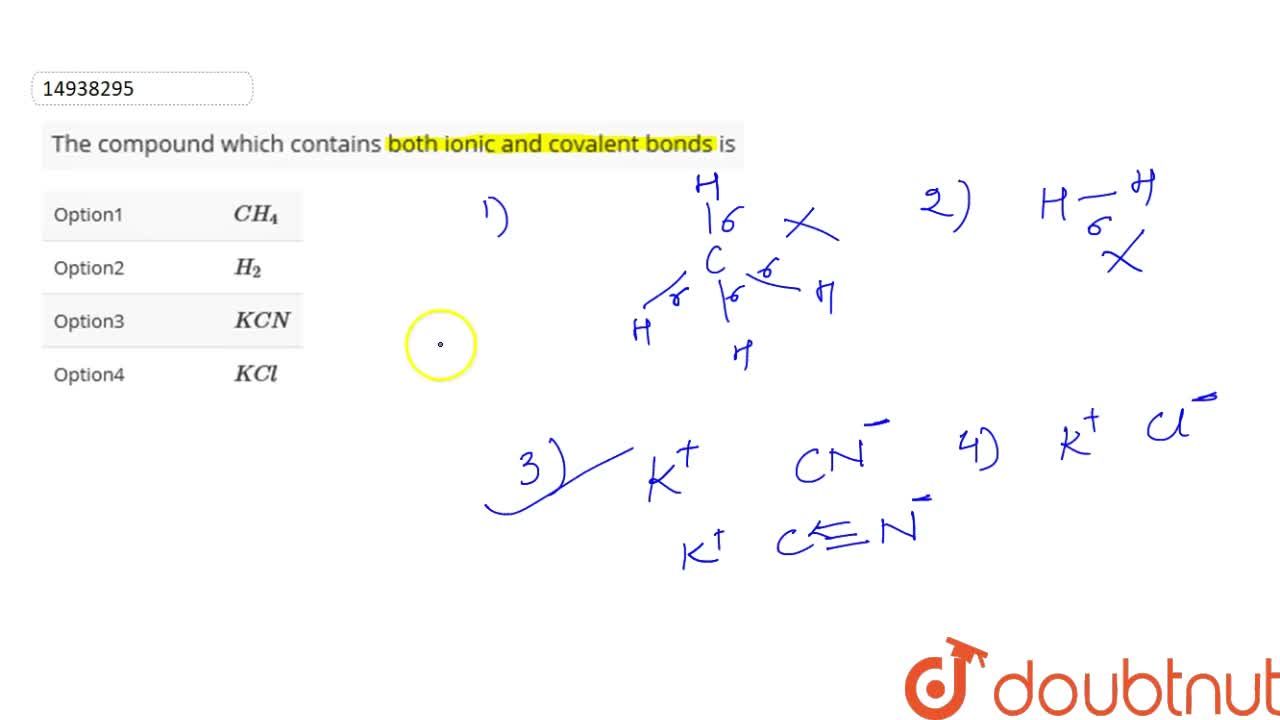
The compound which contains both ionic and covalent bonds is. Chemical bonding is the process that links elements together to form compounds.
Which Of The Following Contains Both Ionic And Covalent Bonds. So if you have a metal and at least two non metals then you�re going to have both ionic and covalin bonds. Then equal sharing of electrons occurs and a nonpolar bond is formed. Some compounds contain both ionic and covalent bonds. Which substance contains both ionic and covalent bonds?
 Which Of The Following Compounds Of Chlorine Contains Both Ionic And Covalent Bonds - Brainly.in From brainly.in
Which Of The Following Compounds Of Chlorine Contains Both Ionic And Covalent Bonds - Brainly.in From brainly.in
Related Post Which Of The Following Compounds Of Chlorine Contains Both Ionic And Covalent Bonds - Brainly.in :
Naclo contains both ionic and covalent bonding. In n 2 o 5, the combining atoms are nitrogen and oxygen. Thus potassium nitrate contains both ionic and covalent bonds. Of these five compounds or ions were asked to pick the one that contains both covalin and ionic bonds.
Which of the following contains both ionic and covalent bonds?
An ionic compound is made of a metal element and a nonmetal element. Ionic bonds have no definite shape: In covalent compounds, some ionic character exists. An ionic compound is made of a metal element and a nonmetal element. These compounds contain polyatomic ions. A) ccl 4 b) kcl c) mgcl 2 d) nh 4cl 13) oxygen, nitrogen, and fluorine bond with hydrogen to form molecules.
 Source: clutchprep.com
Source: clutchprep.com
C o2 c o 2. Name the following which contains covalent and ionic bond 1. An ionic bond is best described as the attraction that holds the atoms together in a polyatomic ion.
 Source: clutchprep.com
Source: clutchprep.com
Ionic bonds have no definite shape: Which one of the following contains both ionic and covalent bonds? Which of the following contains both ionic and covalent bonds?
 Source: youtube.com
Source: youtube.com
Therefore whatever bonds it makes are ionic. Nonmetal + nonmetal = covalent bond. Which of the following compounds contains both ionic.
 Source: slideplayer.com
Source: slideplayer.com
In this compound, cl and o have a covalent bonding in which the electrons are shared between the two atoms. A) nh4no3 b)ch3och3 c)lif d)cacl2 These species share an ionic bond, while the carbon and oxygen atoms in carbonate are covalently bonded.
 Source: slideserve.com
Source: slideserve.com
Covalent bond is present between chromium and oxygen atoms. Both covalent and hydrogen bonds 29. An ionic bond is a chemical bond between two atoms in which one atom seems to donate its electron to another atom.
 Source: clutchprep.com
Source: clutchprep.com
Nonmetal + nonmetal = covalent bond. Which compound contains both covalent and ionic bonds? 12) which compound contains both covalent and ionic bonds?
 Source: chegg.com
Source: chegg.com
An ionic compound is made of a metal element and a nonmetal element. Difference between ionic and covalent bond. Ionic bonds have no definite shape:
 Source: chegg.com
Source: chegg.com
Ionic bonds have no definite shape: Is made up of ionic bond between metal and non metal. Which following contains both ionic and covalent bonds?

Um so if you�re dealing with covalin bonds you have to have two non metals bonded to each other and then an ionic is going to be a metal plus a nonmetal. Covalent bonds, on the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration. The compound which contains both ionic and covalent bonds is.
 Source: youtube.com
Source: youtube.com
Because potassium chloride has ions of opposite charges, potassium chloride is classified as a salt. 12) which compound contains both covalent and ionic bonds? A) n h 4 c l contains both an iconic and covalent bond.

An ionic compound is made of a metal element and a nonmetal element. In covalent compounds, some ionic character exists. In n 2 o 5, the combining atoms are nitrogen and oxygen.
 Source: toppr.com
Source: toppr.com
A so3 b c2h5oh c mgf2 d h2s e nh4cl Which of the following compounds contains both ionic and covalent bonds? Bonds formed from covalent bonding have a definite shape:
 Source: doubtnut.com
Source: doubtnut.com
Which substance contains both ionic and covalent bonds? Composed of more than one nonmetal together. In covalent compounds, some ionic character exists.
 Source: brainly.in
Source: brainly.in
An ionic compound is made of a metal element and a nonmetal element. C 6h 5c l c 6 h 5 c l. Is made up of ionic bond between metal and non metal.
 Source: toppr.com
Source: toppr.com
Low melting point and boiling point Compound of potassium in which both covalent and ionic bonds are present is where ionic bond is present between potassium and chromate ions. Which kinds of bonds are found in a sample of h2o(s)?
 Source: quora.com
Source: quora.com
Ionic bonds have no definite shape: The ammonium ion is polyatomic, which means it forms ionic salts. Let’s first differentiate a covalent bond and an ionic bond.
 Source: toppr.com
Source: toppr.com
C o2 c o 2. Which one of the following contains both ionic and covalent bonds? Bonds formed from covalent bonding have a definite shape:
 Source: fornoob.com
Source: fornoob.com
Covalent bonds, on the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration. Both ionic and hydrogen bonds d. Made up of ionic bond and is made up of covalent bond between two non metals nitrogen and oxygen.
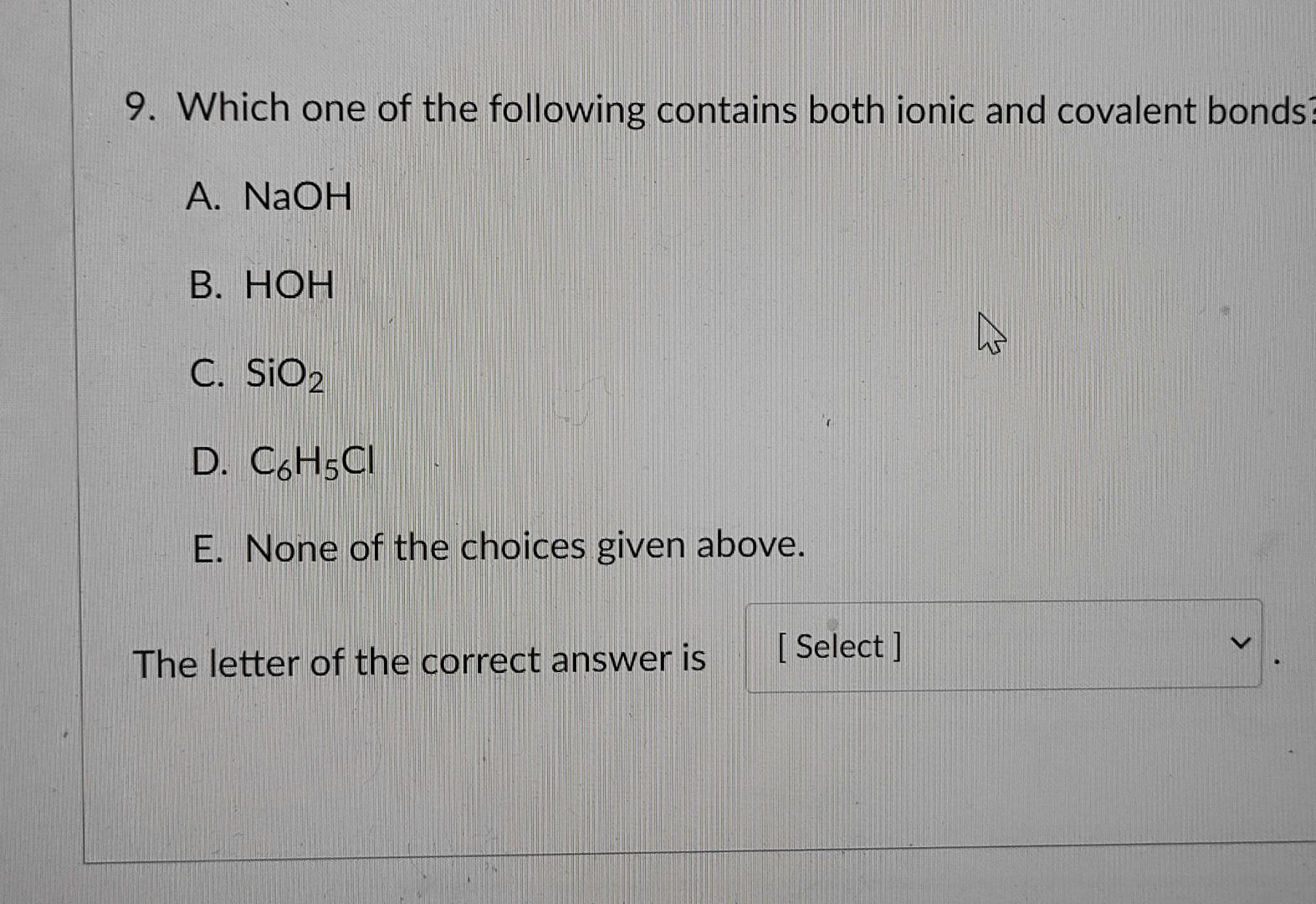
Which one of the following contains both ionic and covalent bonds? Sf 6 cai 2 cos baso 4 none of the above contain both ionic and covalent bonds. Covalent bonds, on the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration.
 Source: youtube.com
Source: youtube.com
Covalent bonds, on the other hand, appear to involve two atoms sharing electrons reach a more stable electron configuration. Sf 6 cai 2 cos baso 4 none of the above contain both ionic and covalent bonds. Compound of potassium in which both covalent and ionic bonds are present is where ionic bond is present between potassium and chromate ions.
Also Read :





