Ionic compounds typically have high melting and boiling points, and are hard and brittle. One may also ask, which compound has the highest boiling point?
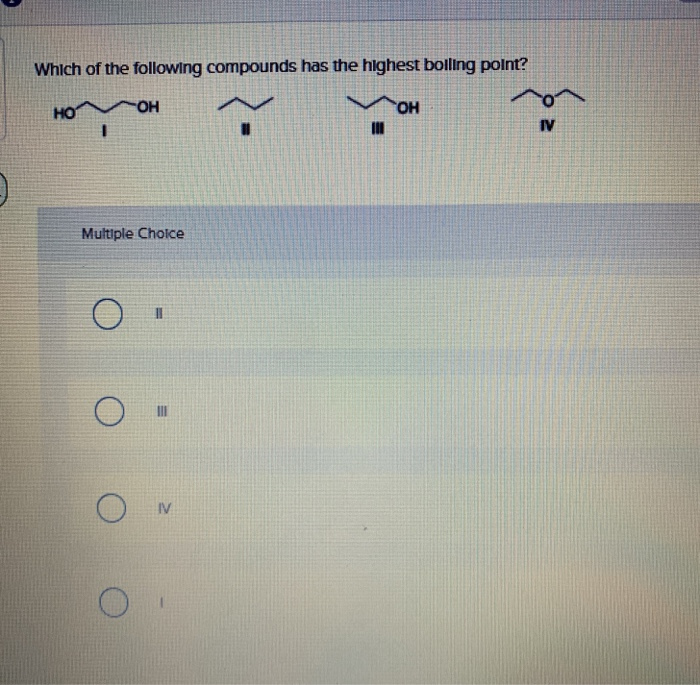
Which Of The Following Compounds Has The Highest Boiling Point. A) i > ii > iii > iv c) iii > ii > iv > i. Which of the following compounds has the highest boiling point? The vander waals dispersion forces increase as the length of the hydrocarbon chain increases. It has the highest boiling points next comes methanol ch4o or ch3oh.
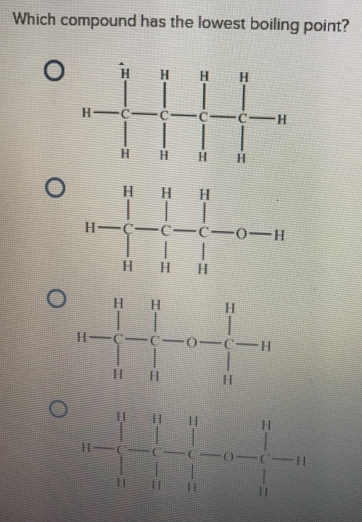 Which Compound Has The Lowest Boiling Poin… | Clutch Prep From clutchprep.com
Which Compound Has The Lowest Boiling Poin… | Clutch Prep From clutchprep.com
Related Post Which Compound Has The Lowest Boiling Poin… | Clutch Prep :
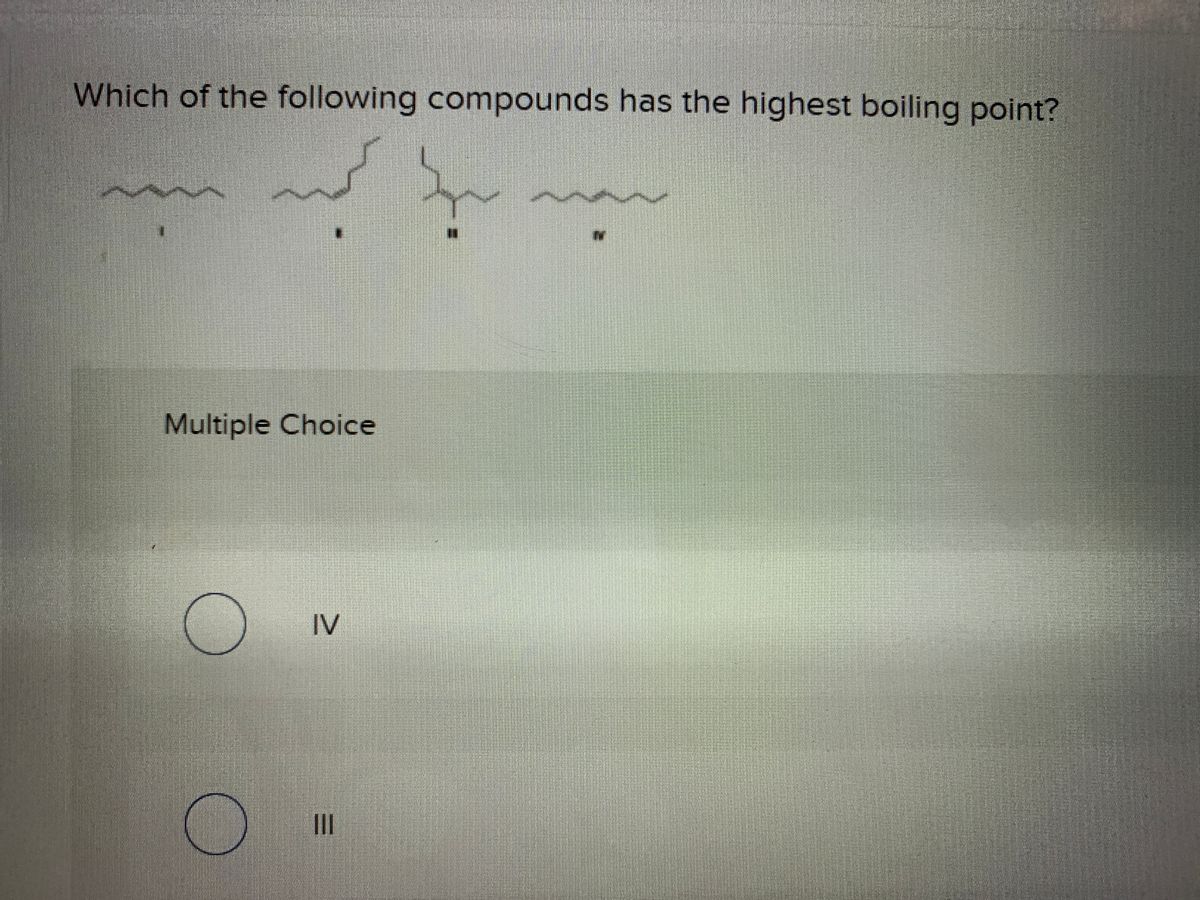
One may also ask, which compound has the highest boiling point? So, $ch _{3} ch _{2} ch _{2} ch _{2} cl$ has highest boiling point. When an array is sorted from highest to lowest, it is said to be in ________ order. Size (the heavier, the stronger the force)
Rank the following compounds according to their boiling point rank the following compounds in order of basicity:
Which solution has the highest boiling point quizlet? It has the highest boiling point that is 77−78 ∘c.(173 ∘f) Click to see full answer. We are asked which of the following compounds has the highest boiling point. It has the highest boiling points next comes methanol ch4o or ch3oh. We’re being asked to identify the compound with the highest boiling point among the given choices.
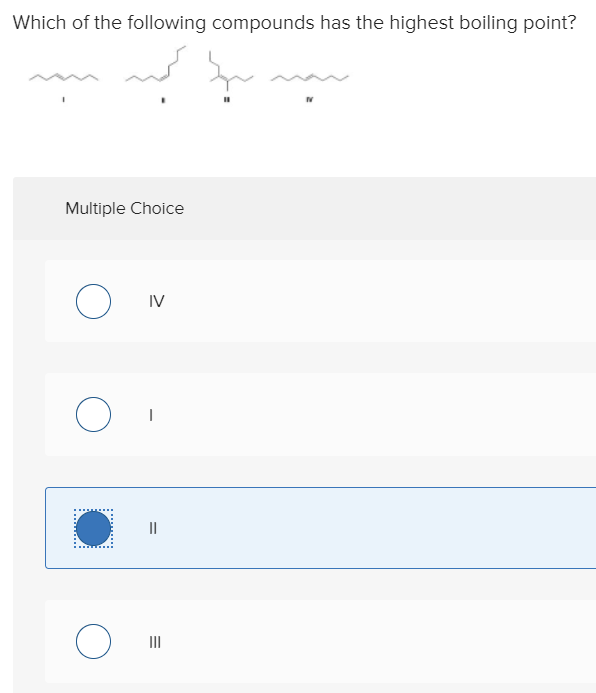 Source: chegg.com
Source: chegg.com
A) i > ii > iii > iv c) iii > ii > iv > i. We review their content and use your feedback to keep the quality high. Which of the following compounds has the highest boiling point?
 Source: neetlab.com
Source: neetlab.com
When an array is sorted from highest to lowest, it is said to be in ________ order. Ch 3 cho is more polar than ch 3 och 3 and so ch3cho has stronger intermolecular dipole − dipole attraction than ch 3 och 3 ch 3 ch 2 ch 3 has only weak van der waals force. Besides, which compound has highest boiling point?
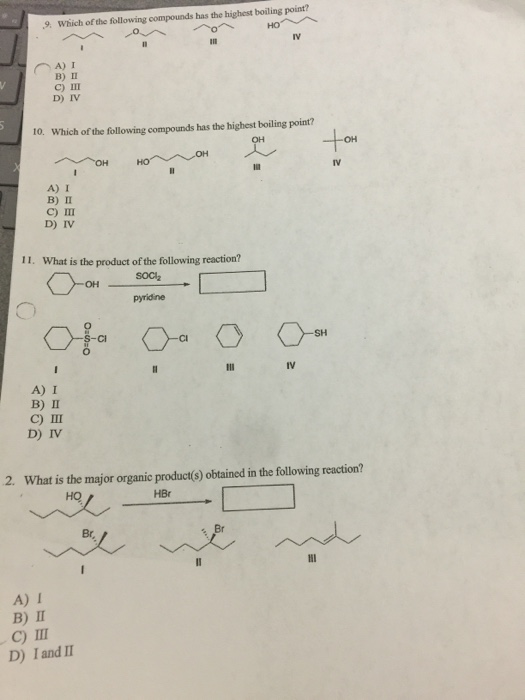 Source: chegg.com
Source: chegg.com
All molecules possess van der waals forces. Which one of the following compounds will have the highest boiling point? It has the highest boiling points next comes methanol ch4o or ch3oh.
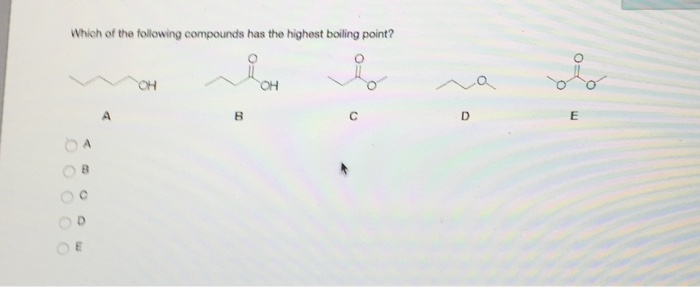
Besides, which compound has highest boiling point? Which of the following compounds has the highest boiling point ?class:11subject: Has planar structure (ring) ii.
 Source: ask.learncbse.in
Source: ask.learncbse.in
Normal boiling point34.5°c heat of vaporization351 j/g specific heat of (ch3ch2)2o (l) 3.74 j/g • °c specific heat of (ch3ch2)2o (g) 2.35 j/g • °c. A)c2cl6 b)c2br6 c)c2h6 d)c2f6 e)c2i6 i know its e, but. Size (the heavier, the stronger the force)
 Source: bartleby.com
Source: bartleby.com
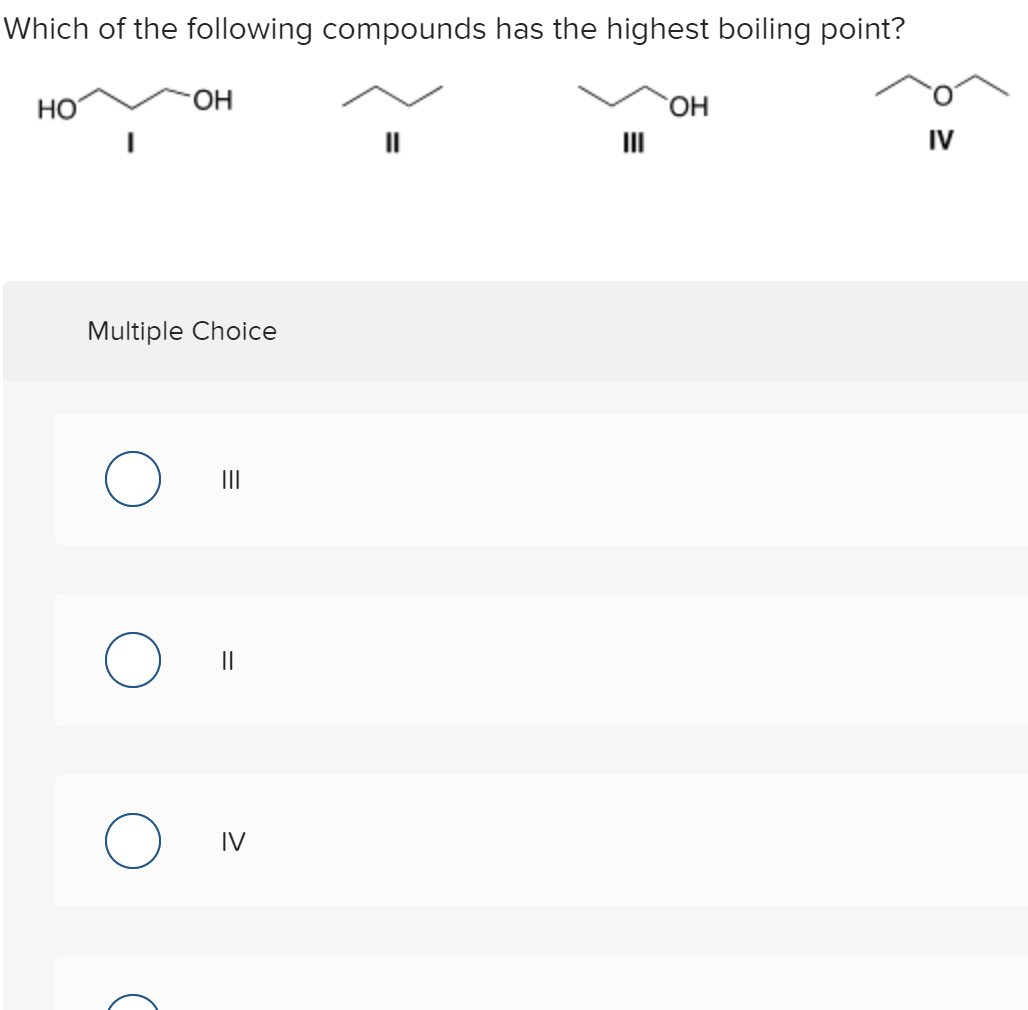
Therefore, it has the highest boiling point. Incorrect question 10 0/5pts which of the following compounds has the highest boiling point? The solution with the highest boiling point is 0.75 m nacl.
 Source: clutchprep.com
Source: clutchprep.com
Which of the following compound has the highest boiling point? It has the highest boiling points next comes methanol ch4o or ch3oh. Hexane has a larger surface area, and therefore the greater dispersion forces and higher boiling point.
 Source: bartleby.com
Source: bartleby.com
Therefore, it has the highest boiling point. No is a polar molecule. Vapour pressure is high in case of branched chains because of less intermolecular forces present in the compound and therefore, boiling point is lower.
 Source: chegg.com
Source: chegg.com
Size (the heavier, the stronger the force) One may also ask, which compound has the highest boiling point? All molecules possess van der waals forces.
 Source: toppr.com
Source: toppr.com
Experts are tested by chegg as specialists in their subject area. It is water white liquid with a sharp odor. Alkanesbook:r sharmaboard:iit jeeyou can ask any doubt fro.
 Source: oneclass.com
Source: oneclass.com
Therefore, ch3ch2oh has the higher boiling point. Ionic compounds typically have high melting and boiling points, and are hard and brittle. This problem has been solved!
 Source: chegg.com
Source: chegg.com
Which solution has the highest boiling point quizlet? Asked aug 28, 2019 in chemistry by huhujiji. Straight chain alkyl halides have greater boiling point than their isomers.
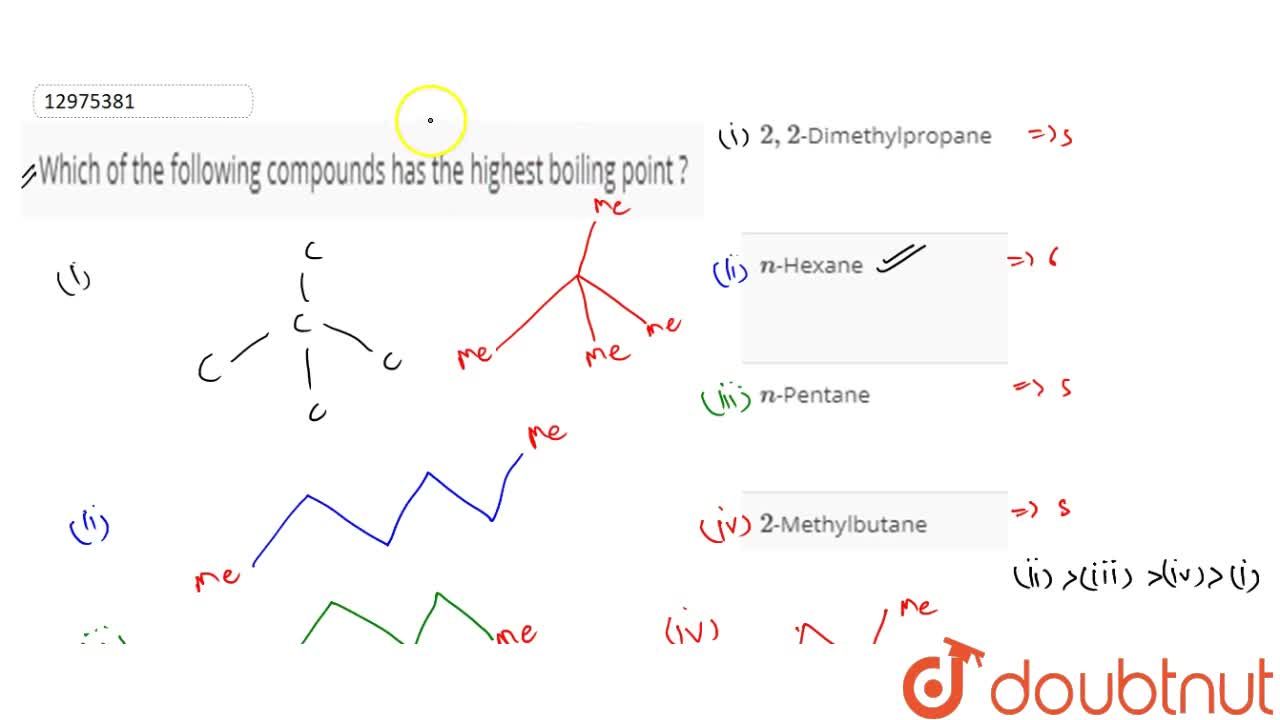 Source: doubtnut.com
Source: doubtnut.com
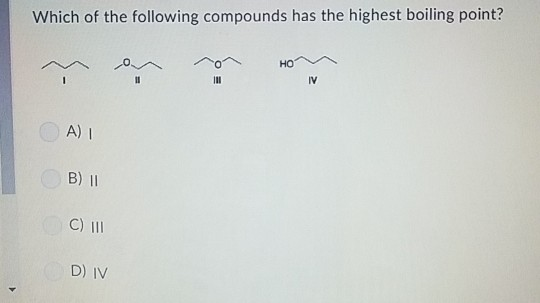
Iv which of the following compounds has the highest boiling point. Rank the boiling points of the following compounds from lowest to highest: It has the highest boiling points next comes methanol ch4o or ch3oh.
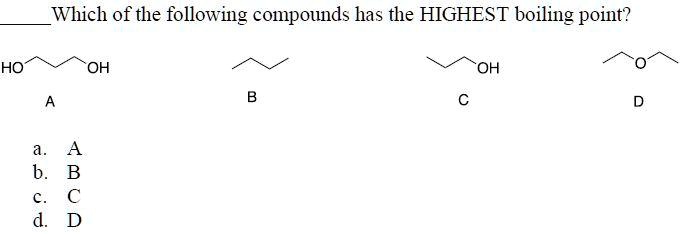 Source: numerade.com
Source: numerade.com
Which substance has the higher boiling point no or n2? A) i > ii > iii > iv c) iii > ii > iv > i. Besides, which compound has highest boiling point?
 Source: brainly.com
Source: brainly.com
A) i > ii > iii > iv c) iii > ii > iv > i. Which substance has the higher boiling point no or n2? These thing will be explained in detail later in this tutorial.
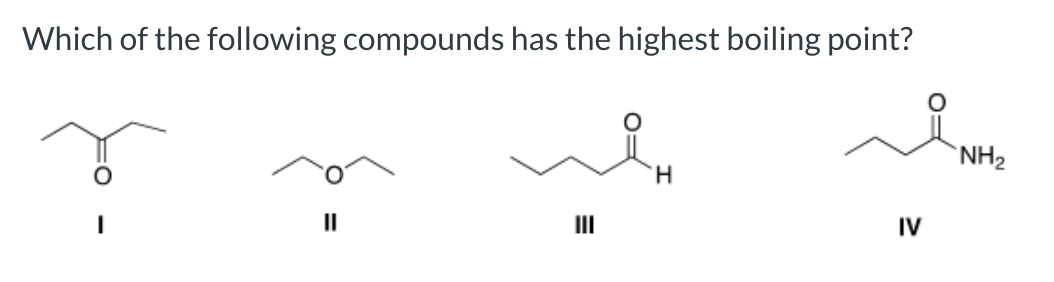 Source: bartleby.com
Source: bartleby.com
Which of the following compounds has the highest boiling point? Therefore, ch3ch2oh has the higher boiling point. Which substance has the higher boiling point no or n2?
![Solved] Which Ofthe Following Compounds Has The Highest Boiling Point? A6 "A At This Question Was Created From Quiz_ Module 8 Homework - Review | Course Hero](https://www.coursehero.com/qa/attachment/2887825/ “Solved] Which Ofthe Following Compounds Has The Highest Boiling Point? A6 "A At This Question Was Created From Quiz_ Module 8 Homework - Review | Course Hero”) Source: coursehero.com
It has the highest boiling points next comes methanol ch4o or ch3oh. One may also ask, which compound has the highest boiling point? A) i b) ii c) iii d) iv.
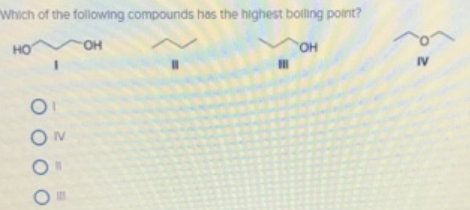 Source: clutchprep.com
Source: clutchprep.com
Methane and ethanoic acid are belongs to two types of organic compounds and according to the type of type, there is a considerable boiling point difference. Which of the following compounds would have the highest boiling point? Ethanol has the highest boiling point (c2h5oh) because of higher description or vander waal.
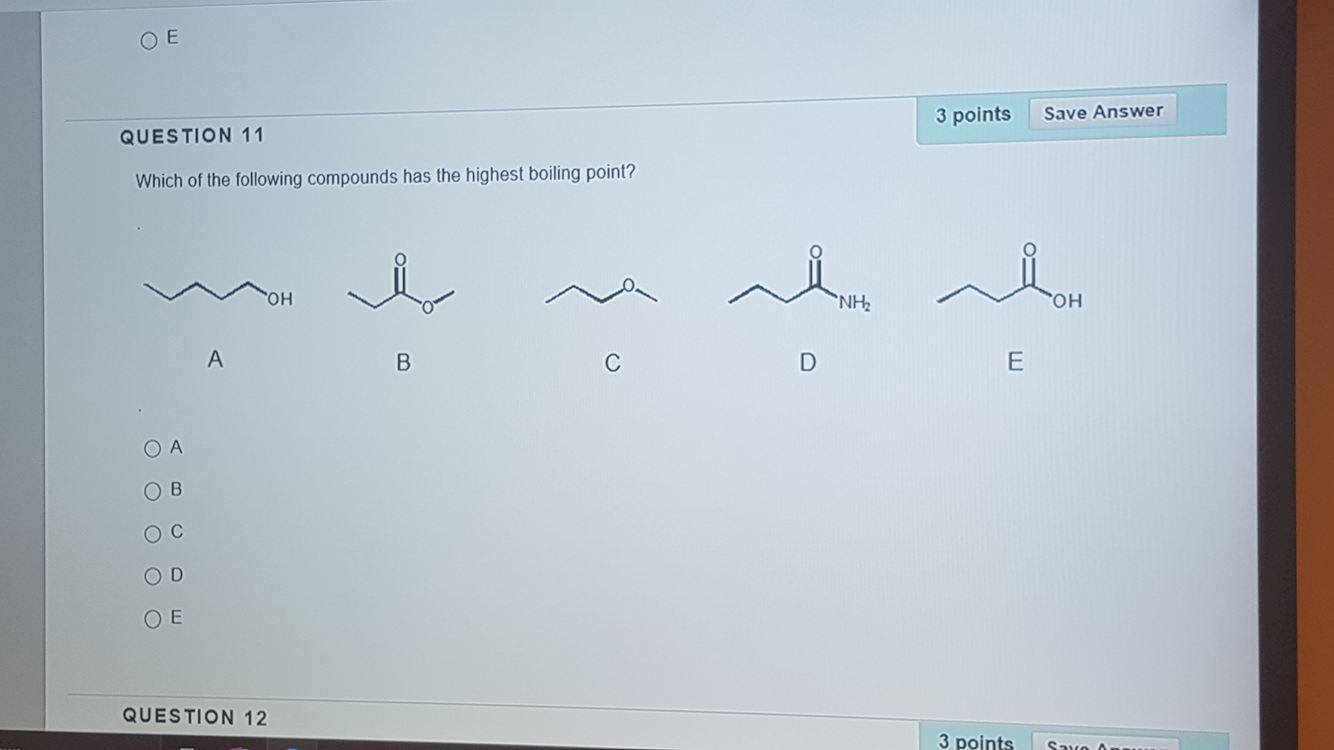 Source: chegg.com
Source: chegg.com
All the given compounds are alkanes so the only present imf is van der waals forces. Which of the following compounds would have the highest boiling point? Ethanoic acid has a higher boiling point than water.
Also Read :





