If both the ch3 group on carbon are in one plane then cis isomer if not the trans isomer. The former is solid at room temperature (melting point = 43 o c) and the latter is found to be liquid, with a melting point of 13.4 o.
Which Of The Following Compounds Can Exhibit Cis Trans Isomerism. (i) the compounds must be cyclic in nature and have flat planar structure. In cyclic compounds, substituents attached to a ring system give rise to geometric isomers. The dipole moment is greater in the case of cis isomers. A) [cu(co)5cl]+ b) [co(h2o)3(co)3] 3+
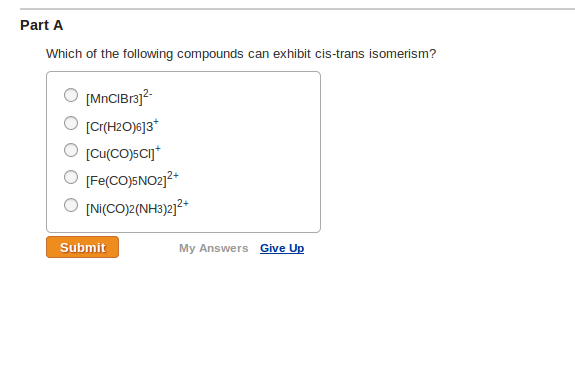 Solved Part A Which Of The Following Compounds Can Exhibit | Chegg.com From chegg.com
Solved Part A Which Of The Following Compounds Can Exhibit | Chegg.com From chegg.com
Related Post Solved Part A Which Of The Following Compounds Can Exhibit | Chegg.com :
So from this, we can say that yes, can for mrs entrance i summers because each side has a hydrogen. Due to restricted rotation about double bond, the alkene shows geometrical isomerism because the relative position of atoms or groups attached to the carbon atoms of the double bond get fixed. Please log in or register to add a comment. C b r 2 = c h 2.
If same group or atom attached with double bond bearing carbon, then alkene doesnt show geometrical isomerism.
Thus, it can be noted that trans isomers generally have higher melting points than their cis counterparts. The dipole moment is greater in the case of cis isomers. If both the ch3 group on carbon are in one plane then cis isomer if not the trans isomer. A) [cu(co)5cl]+ b) [co(h2o)3(co)3] 3+ In cyclic compounds, substituents attached to a ring system give rise to geometric isomers. C b r 2 = c h 2.
 Source: toppr.com
Source: toppr.com
The dipole moment is greater in the case of cis isomers. However, even though there is restricted rotation for triple bond, alkynes do not exhibit geometrical isomerism. Thus, it can be noted that trans isomers generally have higher melting points than their cis counterparts.
 Source: youtube.com
Source: youtube.com
Thus, it can be noted that trans isomers generally have higher melting points than their cis counterparts. Only first option will show cis trans isomerism. In cyclic compounds, substituents attached to a ring system give rise to geometric isomers.

Consider carbon carbon double in plane. The former is solid at room temperature (melting point = 43 o c) and the latter is found to be liquid, with a melting point of 13.4 o. The geometrical isomerism arises due to restricted rotation of double bond.
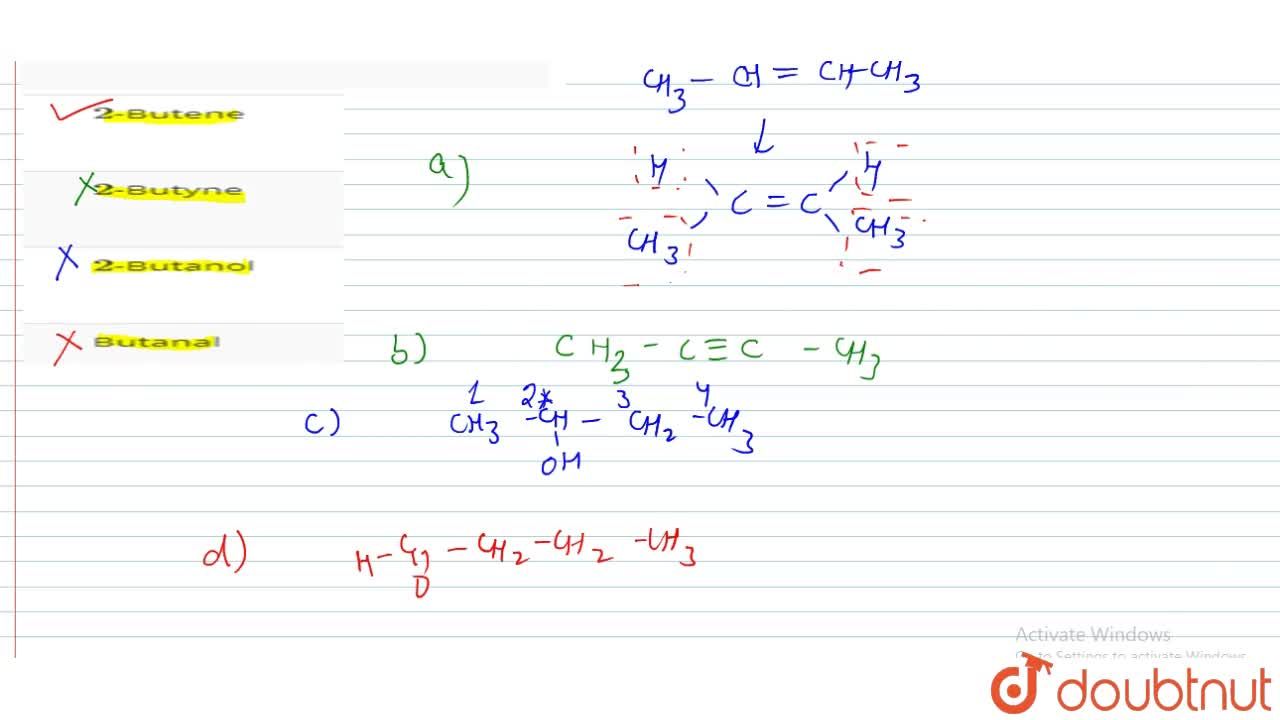 Source: doubtnut.com
Source: doubtnut.com
So the site is that had it and that�s that has a hydrogen and each side has a higher priority groups. The former is solid at room temperature (melting point = 43 o c) and the latter is found to be liquid, with a melting point of 13.4 o. The compounds with each doubly bonded carbon attached to two different groups (like cab=cab, cab=ccd) exhibit geometrical isomerism i.e., cis and trans forms.
 Source: chegg.com
Source: chegg.com
The cis and trans isomers have different physical and chemical properties. If both the ch3 group on carbon are in one plane then cis isomer if not the trans isomer. If same group or atom attached with double bond bearing carbon, then alkene doesnt show geometrical isomerism.
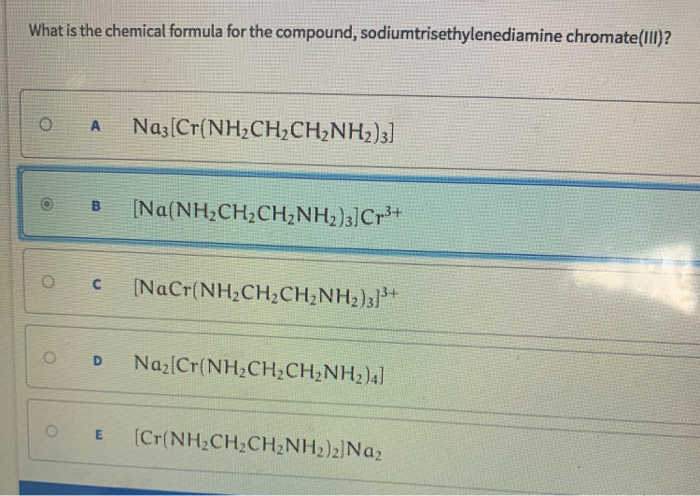 Source: chegg.com
Source: chegg.com
The letters ({\rm{e}}) and ({\rm{z}}) are not used in cyclic alkanes. Only first option will show cis trans isomerism. So from this, we can say that yes, can for mrs entrance i summers because each side has a hydrogen.
 Source: youtube.com
Source: youtube.com
C b r 2 = c h 2. C b r 2 = c h 2. A) [cu(co)5cl]+ b) [co(h2o)3(co)3] 3+
 Source: youtube.com
Source: youtube.com
Consider carbon carbon double in plane. The following pairs of newman projections represent the same compound but in differing conformations. Thus, it can be noted that trans isomers generally have higher melting points than their cis counterparts.
 Source: chegg.com
Source: chegg.com
The geometrical isomerism arises due to restricted rotation of double bond. The letters ({\rm{e}}) and ({\rm{z}}) are not used in cyclic alkanes. The cis and trans isomers have different physical and chemical properties.
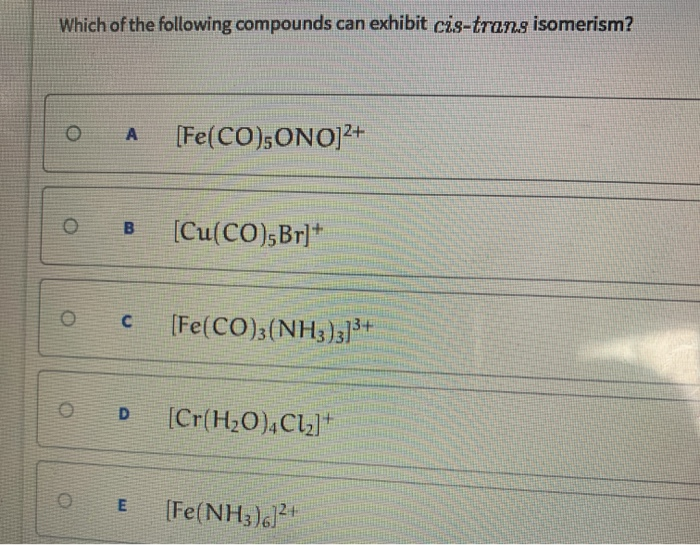 Source: chegg.com
Source: chegg.com
If both the ch3 group on carbon are in one plane then cis isomer if not the trans isomer. The compounds possessing aromatic character show the following characteristics: So the site is that had it and that�s that has a hydrogen and each side has a higher priority groups.
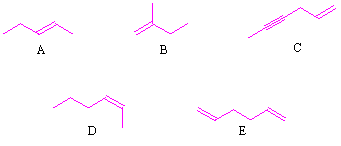 Source: chegg.com
Source: chegg.com
Due to restricted rotation about double bond, the alkene shows geometrical isomerism because the relative position of atoms or groups attached to the carbon atoms of the double bond get fixed. The cis and trans isomers have different physical and chemical properties. The substituents will either be on the same side of the ring or the opposite side of the alkane ring.
 Source: toppr.com
Source: toppr.com
So the site is that had it and that�s that has a hydrogen and each side has a higher priority groups. The compounds with each doubly bonded carbon attached to two different groups (like cab=cab, cab=ccd) exhibit geometrical isomerism i.e., cis and trans forms. Due to restricted rotation about double bond, the alkene shows geometrical isomerism because the relative position of atoms or groups attached to the carbon atoms of the double bond get fixed.
 Source: youtube.com
Source: youtube.com
The former is solid at room temperature (melting point = 43 o c) and the latter is found to be liquid, with a melting point of 13.4 o. If playback doesn�t begin shortly, try. However, even though there is restricted rotation for triple bond, alkynes do not exhibit geometrical isomerism, since the triply bonded carbons are attached to one group each only.
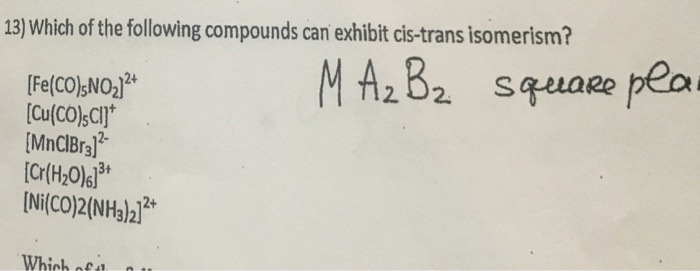
However, even though there is restricted rotation for triple bond, alkynes do not exhibit geometrical isomerism, since the triply bonded carbons are attached to one group each only. Thus it doesn�t fulfill the criteria to show geometrical isomerism. The substituents will either be on the same side of the ring or the opposite side of the alkane ring.
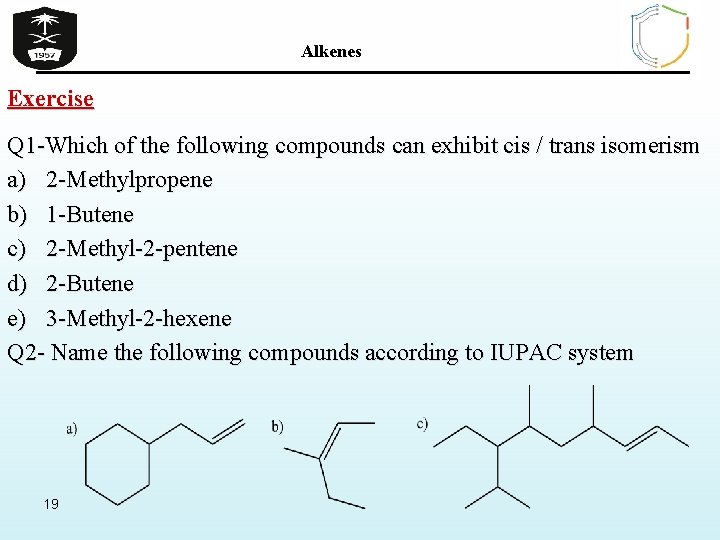 Source: slidetodoc.com
Source: slidetodoc.com
In cyclic compounds, substituents attached to a ring system give rise to geometric isomers. The former is solid at room temperature (melting point = 43 o c) and the latter is found to be liquid, with a melting point of 13.4 o. So the site is that had it and that�s that has a hydrogen and each side has a higher priority groups.
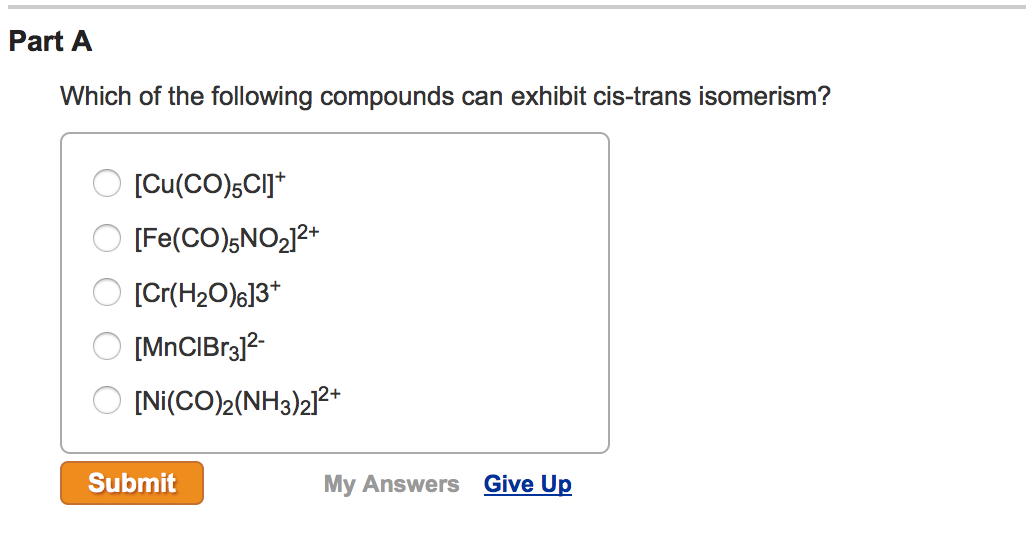 Source: chegg.com
Source: chegg.com
(i) the compounds must be cyclic in nature and have flat planar structure. Thus it doesn�t fulfill the criteria to show geometrical isomerism. The geometrical isomerism arises due to restricted rotation of double bond.

The compounds with each doubly bonded carbon attached to two different groups (like cab=cab, cab=ccd) exhibit geometrical isomerism i.e., cis and trans forms. The trans isomers generally gave higher melting points than the cis ones. Thus, it can be noted that trans isomers generally have higher melting points than their cis counterparts.
 Source: chegg.com
Source: chegg.com
Thus, it can be noted that trans isomers generally have higher melting points than their cis counterparts. The letters ({\rm{e}}) and ({\rm{z}}) are not used in cyclic alkanes. The trans isomers generally gave higher melting points than the cis ones.
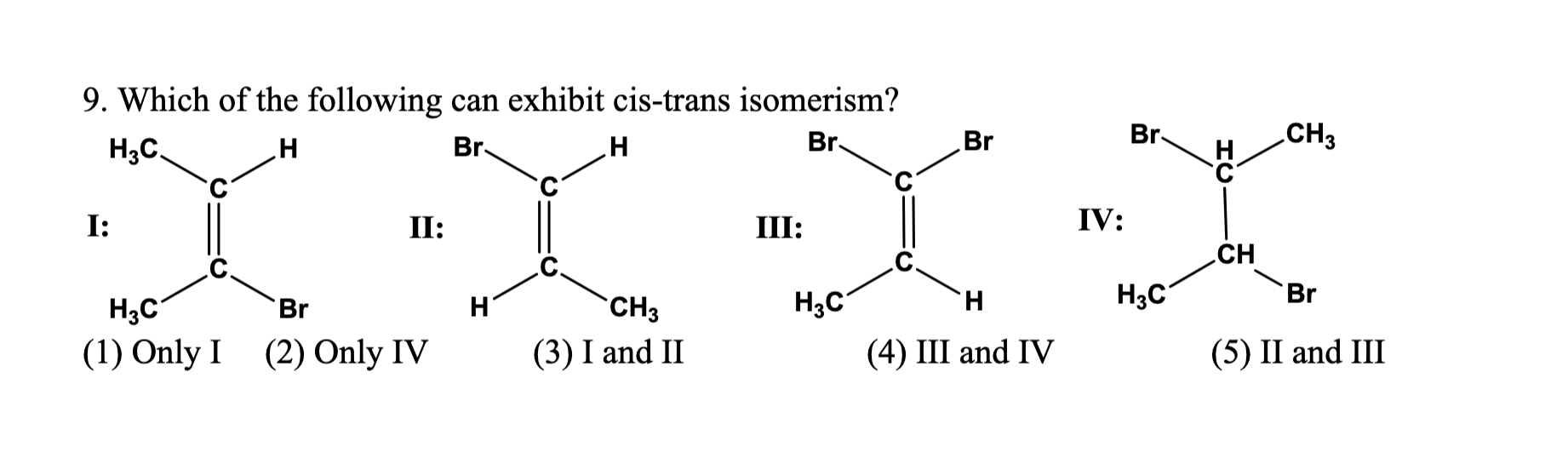 Source: chegg.com
Source: chegg.com
The former is solid at room temperature (melting point = 43 o c) and the latter is found to be liquid, with a melting point of 13.4 o. However, even though there is restricted rotation for triple bond, alkynes do not exhibit geometrical isomerism. The trans isomers generally gave higher melting points than the cis ones.
 Source: clutchprep.com
Source: clutchprep.com
The trans isomers generally gave higher melting points than the cis ones. If same group or atom attached with double bond bearing carbon, then alkene doesnt show geometrical isomerism. If playback doesn�t begin shortly, try.
Also Read :





