Click card to see definition 👆. Nickel carbonate is soluble in dilute acids and ammonia, and insoluble in hot water.
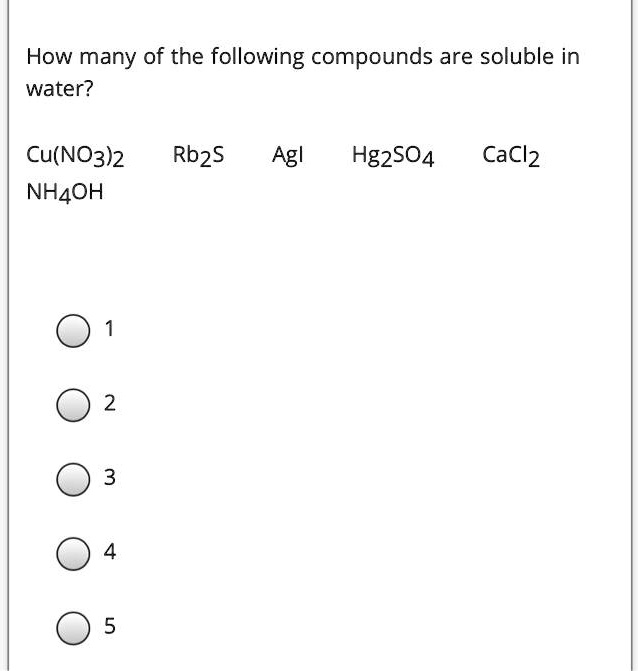
Which Of The Following Compounds Are Soluble In Water. So according to rule six compound formed by s two minus and pb two plus will be insoluble. Which state symbol is used for a soluble substance? (vi) pentanol is partially soluble in water. So we need to reference our scalability rules.
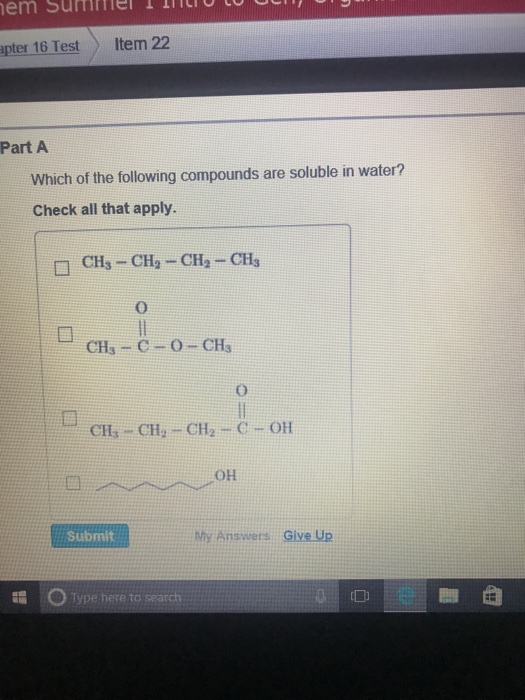
Related Post Solved Which Of The Following Compounds Are Soluble In | Chegg.com :
A polar molecule is one that�s neutral, or uncharged, but has an asymmetric internal distribution of. The lattric enthalpies of agbr and agl are even higher because of greater number of electrons in their anions. It can react violently with iodine, hydrogen sulfide, or a mixture of barium oxide and air. Nh 4 br, cubr 2 , agl, pb(no 3 ) 2 , cuco 3 , caso 4 learn this topic by watching solubility rules concept videos
The chemicals have to be exposed to their boiling point to fully dissolve.
Y different a) 1 b) 2 c) 3 d) 4 e) no solids will precipitate 8). So according to rule six compound formed by s two minus and pb two plus will be insoluble. Since this is not the case here, sodium chloride will be soluble. Which compounds are soluble in water. If there two rules appear to contradict each other, the preceding rule takes precedence. (i) phenol is partially soluble in water.
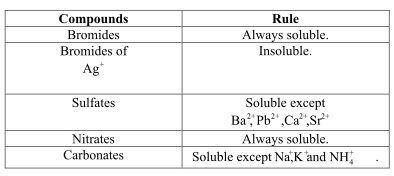 Source: ask.learncbse.in
Source: ask.learncbse.in
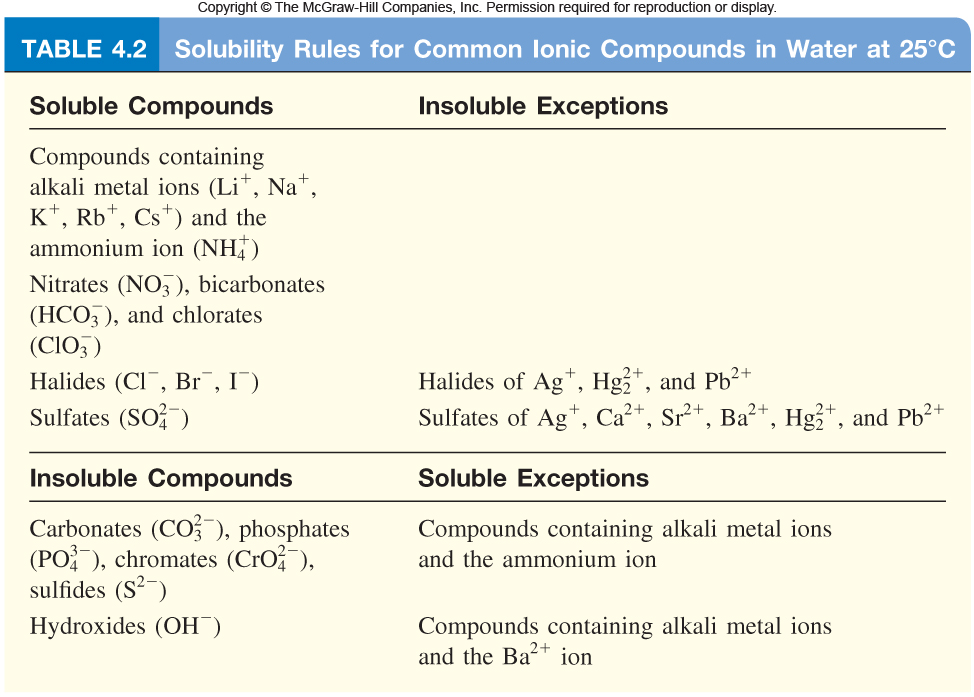
Hence, agci is much less soluble in water than kci. Methanamine (methylamine), ethanamine, propylamine are soluble in water. (c) is soluble in water as compounds containing nitrate ions are water soluble unless mercury cation is one of the insoluble exception.
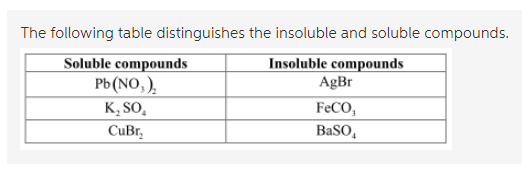 Source: ask.learncbse.in
Source: ask.learncbse.in
Hence, agci is much less soluble in water than kci. 6) all of the following compounds are soluble in water except: For more detailed information of the exact solubility of the compounds, see the solubility table.

The lattric enthalpies of agbr and agl are even higher because of greater number of electrons in their anions. In a base, dichromates convert into chromates and some of the chromates are insoluble in water. A white solid is observed to be insoluble in water, insoluble in excess ammonia.
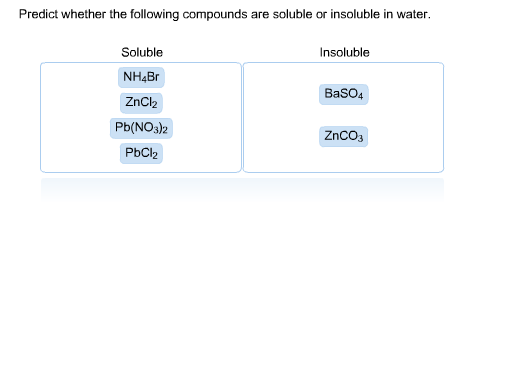 Source: chegg.com
Source: chegg.com
Iodine, cobalt (ii) chloride and sucrose (c12h22o11). * water is sometimes called the universal solvent. * water is good at dissolving ions and polar molecules, but poor at dissolving nonpolar molecules. (d) is soluble in water compounds containing nitrate ions are water soluble unless mercury cation is one of the insoluble exception for sulfates.
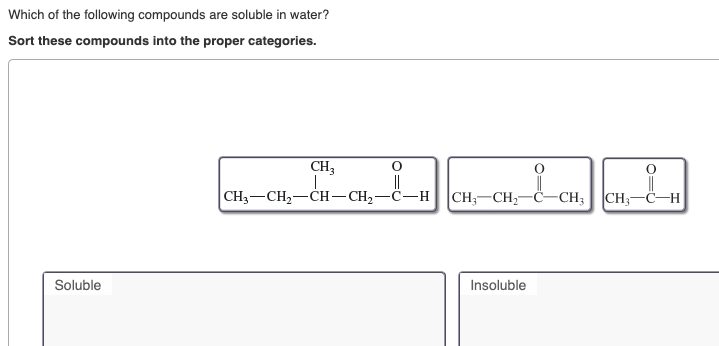
Which state symbol is used for a soluble substance? Which of the following ionic compounds is most soluble in water? So here we�re taking a look at pb two plus and which of the following iron form compounds with pb two plus that will be generally soluble in water.
 Source: lisbdnet.com
Source: lisbdnet.com
A white solid is observed to be insoluble in water, insoluble in excess ammonia. So according to rule six compound formed by s two minus and pb two plus will be insoluble. (ii) toluene is water insoluble.
 Source: clutchprep.com
Source: clutchprep.com
Iodine, cobalt (ii) chloride and sucrose (c12h22o11). Rank the following compounds in order of their solubility in water. Which compound is soluble in cold water?
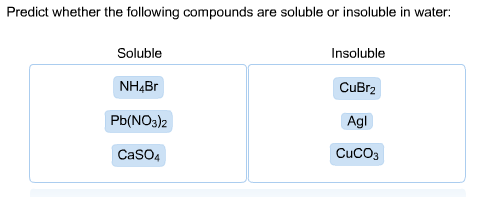 Source: chegg.com
Source: chegg.com
Sodium chloride (nacl) table salt, or sodium chloride (nacl) , the most common ionic compound, is soluble in water (360 g/l). A polar molecule is one that�s neutral, or uncharged, but has an asymmetric internal distribution of. (c) is soluble in water as compounds containing nitrate ions are water soluble unless mercury cation is one of the insoluble exception.
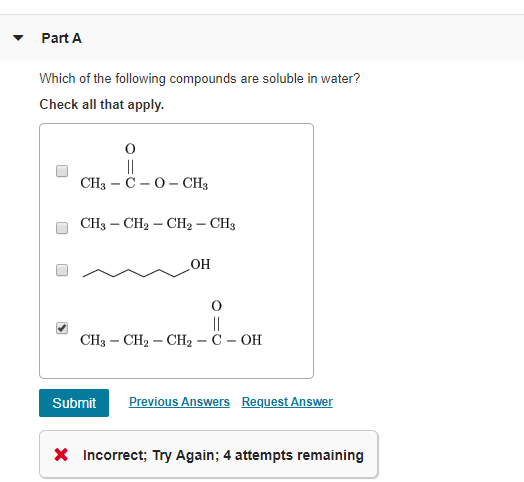 Source: chegg.com
Source: chegg.com
Lead sulfate is insoluble in cold water whereas most of the sulfates are soluble in cold water. The biology project department of biochemistry and molecular biophysics (b) is soluble in water as compounds containing sulfate ions are water soluble.
 Source: transtutors.com
Source: transtutors.com
(v) chloroform is water insoluble. This is nitrobenzene, and it is a covalent compound which is a reason that it is soluble in benzene, alcohol etc but insoluble in water. So according to rule six compound formed by s two minus and pb two plus will be insoluble.
 Source: bartleby.com
Source: bartleby.com
The chemicals have to be exposed to their boiling point to fully dissolve. Consequently, they are les soluble than agci. But solubility is low when alkyl group is large.
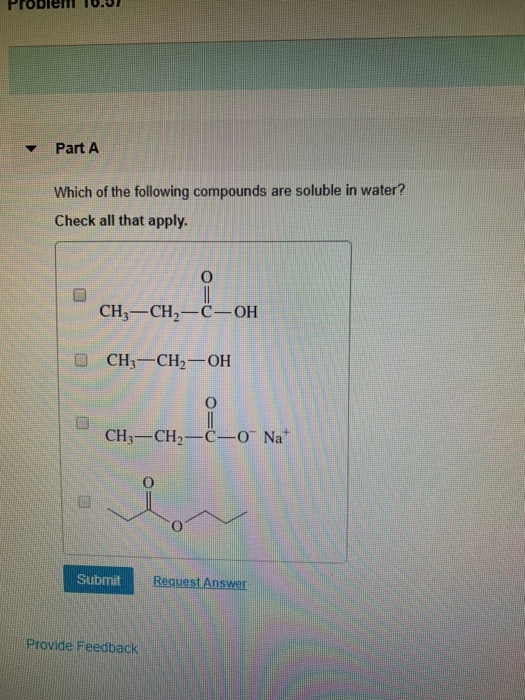 Source: chegg.com
Source: chegg.com
Almost all ionic compounds are soluble in water but there are few exceptions. Which state symbol is used for a soluble substance? So according to rule six compound formed by s two minus and pb two plus will be insoluble.
 Source: numerade.com
Source: numerade.com
The chemicals have to be exposed to their boiling point to fully dissolve. Lead sulfate is insoluble in cold water whereas most of the sulfates are soluble in cold water. Methanamine (methylamine), ethanamine, propylamine are soluble in water.
 Source: transtutors.com
Source: transtutors.com
Methanamine (methylamine), ethanamine, propylamine are soluble in water. The correct answer is option “d”. 6) all of the following compounds are soluble in water except:
 Source: numerade.com
Source: numerade.com
Are the following ionic compounds soluble or insoluble in water? Lead sulfate is insoluble in cold water whereas most of the sulfates are soluble in cold water. Predict whether the following compounds are soluble or insoluble in water:
 Source: ask.learncbse.in
Source: ask.learncbse.in
Methanamine (methylamine), ethanamine, propylamine are soluble in water. Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. Silver nitrate, or agn o3, is soluble because all nitrates are soluble, without exception.

A white solid is observed to be insoluble in water, insoluble in excess ammonia. The halide salts of silver and lead are also insoluble in water. Sodium carbonate () is soluble in water.

Sodium chloride (nacl) table salt, or sodium chloride (nacl) , the most common ionic compound, is soluble in water (360 g/l). Which compound is soluble in cold water? Which state symbol is used for a soluble substance?
 Source: clutchprep.com
Source: clutchprep.com
The correct answer is option “d”. (d) is soluble in water compounds containing nitrate ions are water soluble unless mercury cation is one of the insoluble exception for sulfates. The chemicals have to be exposed to their boiling point to fully dissolve.
 Source: socratic.org
Source: socratic.org
But aniline can form hydrogen bonds. If there two rules appear to contradict each other, the preceding rule takes precedence. Amine which have less molecular mass are soluble in water.
Also Read :




