Which among the following is the most stable carbocation ? Classify the following solvents as nonpolar (np), polar
Which Of The Following Carbocations Is The Most Stable. Nice to be helped this one. Provide the structure of the rearranged carbocation below the original structure. Therefore allylic and benzylic systems are more stable than their saturated counterparts. H c h 2 c h 3.

Related Post Which Of The Following Carbocations Is The Most Stable? - Sarthaks Econnect | Largest Online Education Community :
What is the decreasing order of stability (most stable rarr stable ) of the. Hence, it is much more stable than benzyl carbocation. Videos you watch may be added to the tv�s watch history. This car book today is the most stable mind because this car is the signal providing intel, carved wooden in the signal profiled metal car boudin number of the ordinance are very high.
Hence the correct option is b.
The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer. Hence, it is much more stable than benzyl carbocation. The question is which among the fallen car walk then, is the most stable part. Hence the maximum will be stability. Be aware of the potential for heteroatoms to stabilize carbocations (since they. ( c h 3) 3 c +.
 Source: vedantu.com
Source: vedantu.com
Asked jan 20, 2020 in chemistry by yogitareddy (82.5k points) closed dec 14, 2021. Asked jan 20, 2020 in chemistry by yogitareddy (82.5k points) closed dec 14, 2021. So, we can say that.
 Source: study.com
Source: study.com
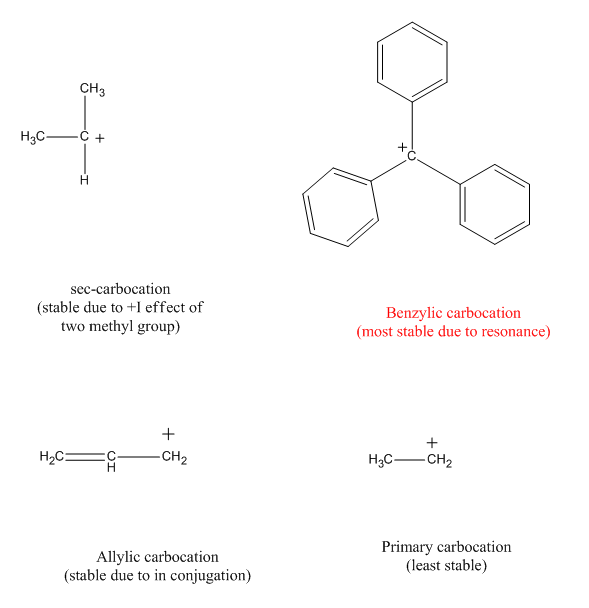
Hence, the most stable carbocation is triphenyl methyl carbocation. Videos you watch may be added to the tv�s watch history. Hence option b is more stable.

So out of the given options, only compound (ii) has a tertiary. Which of the following carbocations will be the most stable? More the number of sigma c − h bonds at alpha position, more the number of hyperconjugating structures and more the stability.
− n o 2 group exhibit − i effect and it decreases with increase in distance. Rank the following carbocations from most to least stable. Which of the following carbocations is expected to be most stable ?
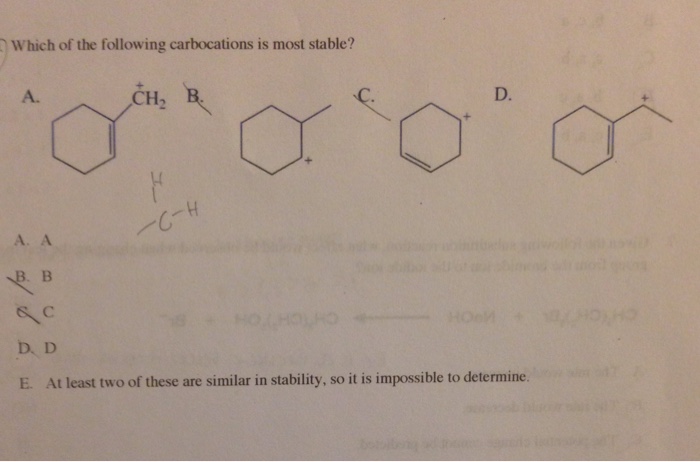
Hence the correct option is b. ( c h 3) 3 c +. Arrange the following carbocations in decreasing order (most stable first) of their stability.
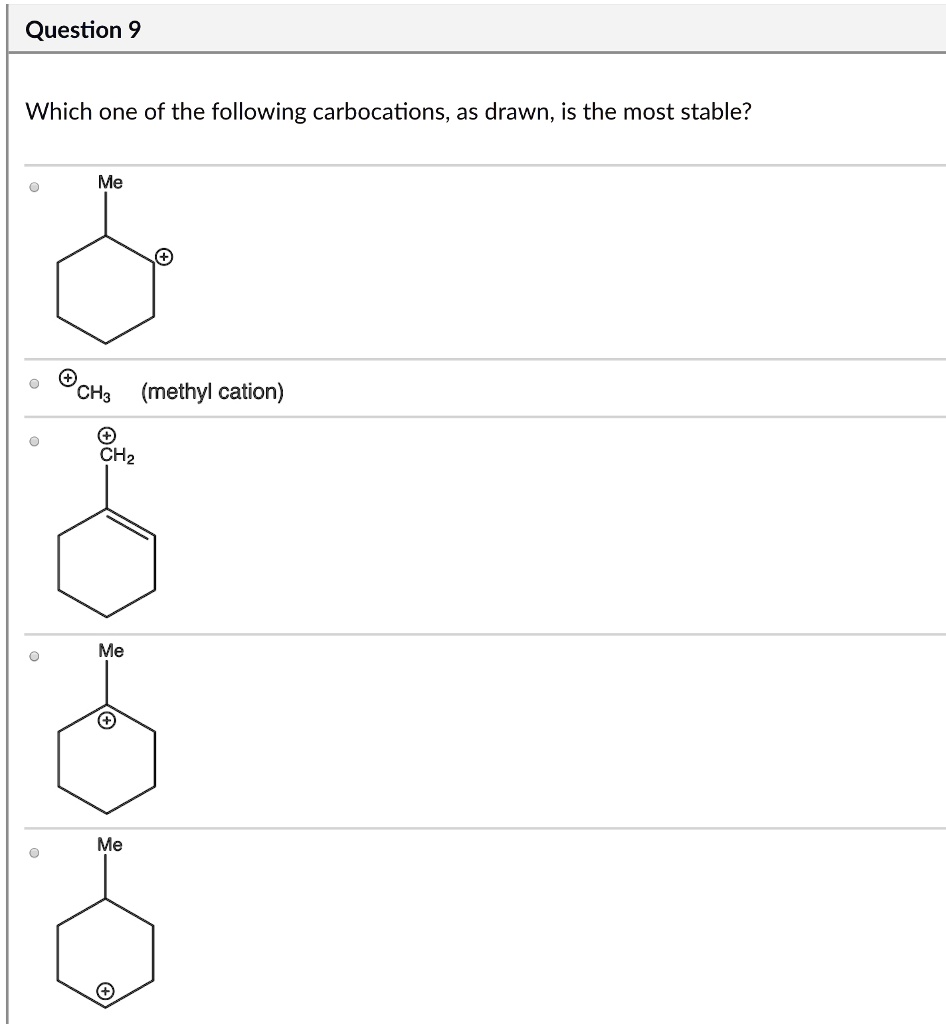 Source: itprospt.com
Source: itprospt.com
Be aware of the potential for heteroatoms to stabilize carbocations (since they. Hence option b is more stable. It is because tertiary carbocations have more inductively donating groups as compared to secondary and primary carbocation.
 Source: toppr.com
Source: toppr.com
Therefore allylic and benzylic systems are more stable than their saturated counterparts. Circle the carbocations in the following group that would be expected to undergo rearrangement. Hence the correct option is b.
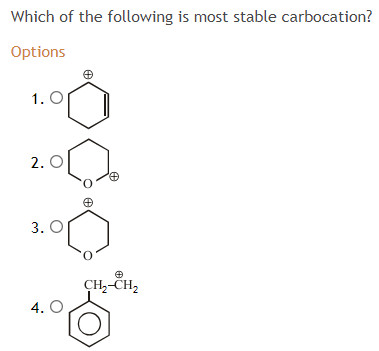 Source: socratic.org
Source: socratic.org
Hence, the most stable carbocation is triphenyl methyl carbocation. More the number of sigma c − h bonds at alpha position, more the number of hyperconjugating structures and more the stability. What is the decreasing order of stability (most stable rarr stable ) of the.
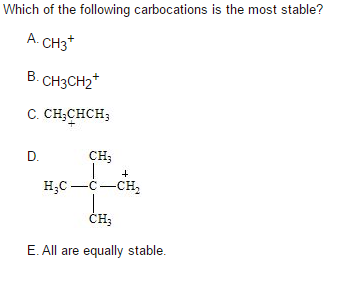 Source: chegg.com
Source: chegg.com
If we know the order of stability of carbocations, we know that tertiary is the most stable, then is secondary, and primary as the least stable. Will be the most stable carbocation amongst the other carbocations given. Classify the following solvents as nonpolar (np), polar
 Source: brainly.in
Source: brainly.in
A tertiary carbocation forms the most quickly because it is the most stable. Methyl carbocation is a very weak electron donating group. But if we have more carbocations of one type, then we have to determine which of them is more stable.
 Source: clutchprep.com
Source: clutchprep.com
There is no resonance nor inductive effect in methyl carbocation and thus it is the least stable among the following. Which of the following carbocations will be the most stable? Arrange the following carbocations in decreasing order (most stable first) of their stability.

Classify the following solvents as nonpolar (np), polar So, we can say that. Hence, it is much more stable than benzyl carbocation.
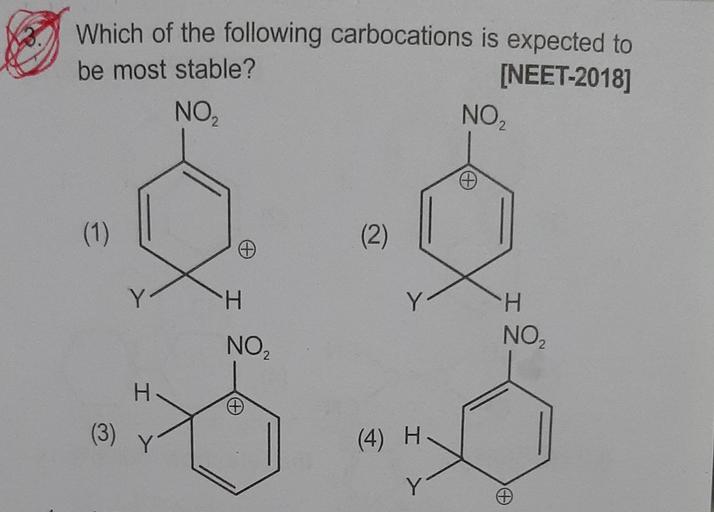 Source: questions-in.kunduz.com
Source: questions-in.kunduz.com
Asked jan 20, 2020 in chemistry by yogitareddy (82.5k points) closed dec 14, 2021. If playback doesn�t begin shortly, try restarting your device. P h3c + p h 3 c +.
 Source: itprospt.com
Source: itprospt.com
Provide the structure of the rearranged carbocation below the original structure. The question is which among the fallen car walk then, is the most stable part. What is the decreasing order of stability (most stable rarr stable ) of the.
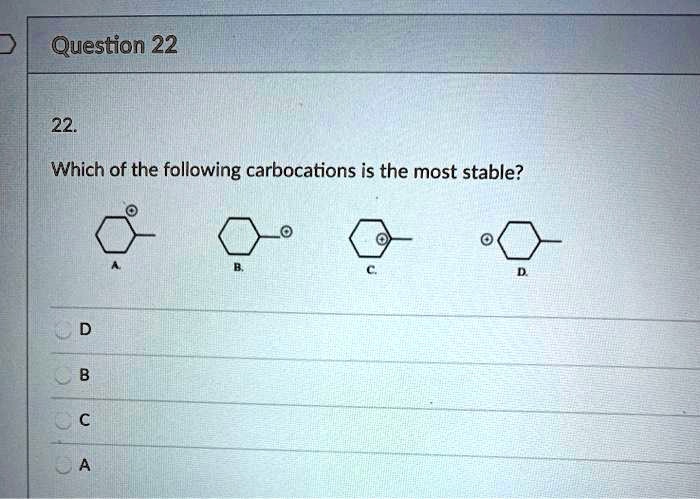 Source: numerade.com
Source: numerade.com
Hence structure (b) which is having nine sigma c − h bonds will be the most stable carbocation. Rank the carbocations in order of decreasing stability. There is no resonance nor inductive effect in methyl carbocation and thus it is the least stable among the following.
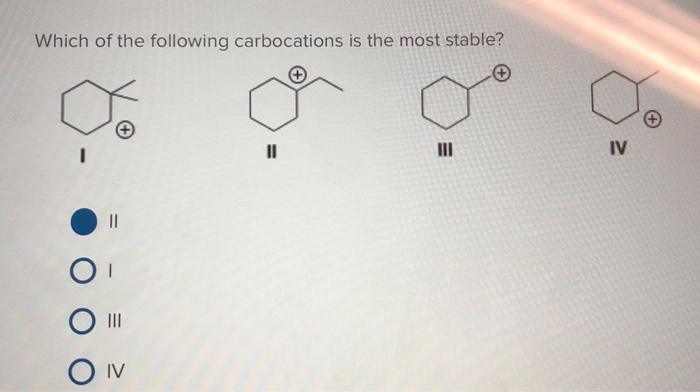 Source: chegg.com
Source: chegg.com
So out of the given options, only compound (ii) has a tertiary. It is because tertiary carbocations have more inductively donating groups as compared to secondary and primary carbocation. The positively charged carbon atom is bonded with two carbon atoms and one hydrogen atom, so we will call it a secondary carbocation and it will be less stable than tertiary carbocation.

(c h 3)2c + h ( c h 3) 2 c h +. Provide the structure of the rearranged carbocation below the original structure. ( c h 3) 3 c +.
 Source: toppr.com
Source: toppr.com
The more alkyl groups that are attached to a positively charged carbon, the more the carbocation is stabilized by hyperconjugation. A tertiary carbocation forms the most quickly because it is the most stable. Rank the following carbocations in order of decreasing stability most stable least stable ch3.
 Source: toppr.com
Source: toppr.com
Guys….to be true 3° is more stable than…tribenzylic methyl carbocation….but there is one family which is more stable than 3° carbocation. Rank the following carbocations in order of decreasing stability most stable least stable ch3. The tertiary carbocations are more stable than secondary because we have three electron donating groups and in secondary it�ll be two and for primary it is one.
 Source: toppr.com
Source: toppr.com
(c h 3)2c + h ( c h 3) 2 c h +. Arrange the following carbocations in decreasing order (most stable first) of their stability. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Also Read :