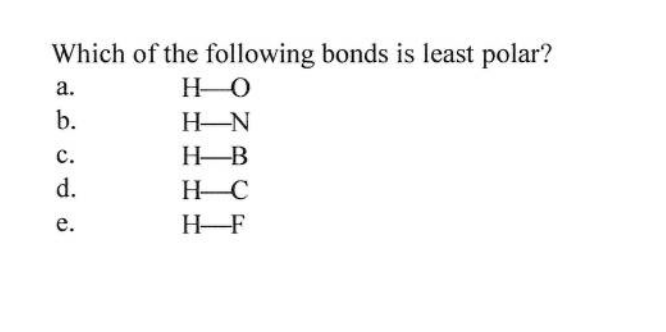
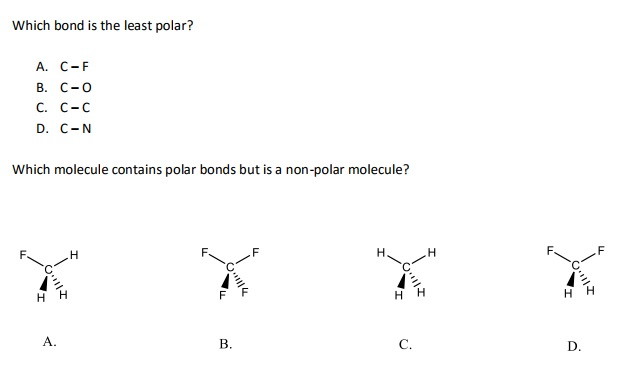
The correct answer is therefore b. So we're gonna be identifying the most polar bond and the least polar bonds.
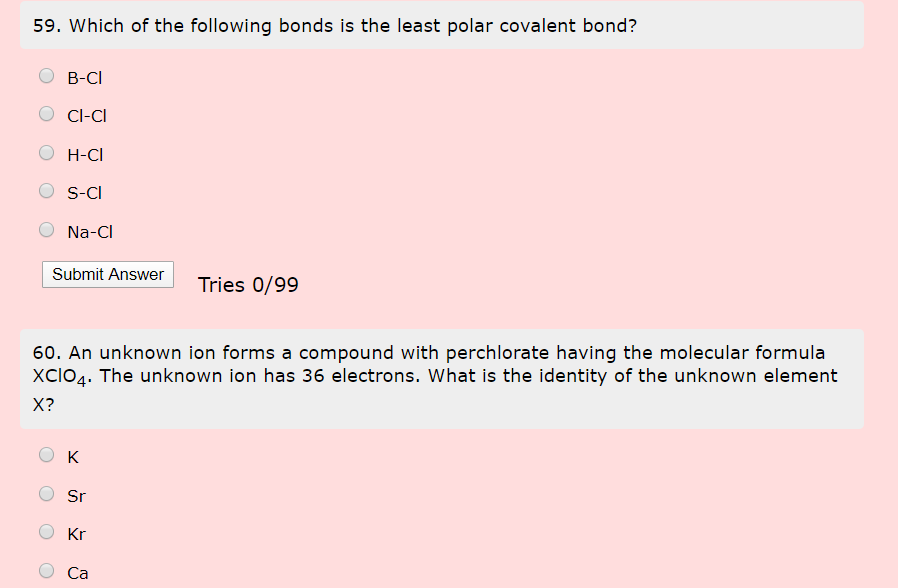
Which Of The Following Bonds Is Least Polar. Polar covalent bonds can be present in a nonpolar molecule. In practice no bond is totally ionic. Which of the following bonds is the least polar? A) rb b) ga c) n d) ar e) i 10.
 Solved É Oso Which Of The Following Bonds Is Least Polar? Ho | Chegg.com From chegg.com
Solved É Oso Which Of The Following Bonds Is Least Polar? Ho | Chegg.com From chegg.com
Related Post Solved É Oso Which Of The Following Bonds Is Least Polar? Ho | Chegg.com :
Covalent compounds are soluble in. Where q the magnitude of the charge on each end of the dipole and r is the distance between the. In which case is the bond polarity incorrect? Polar covalent bonds can be present in a nonpolar molecule.
The electronegativity difference between the two atoms will be calculated.
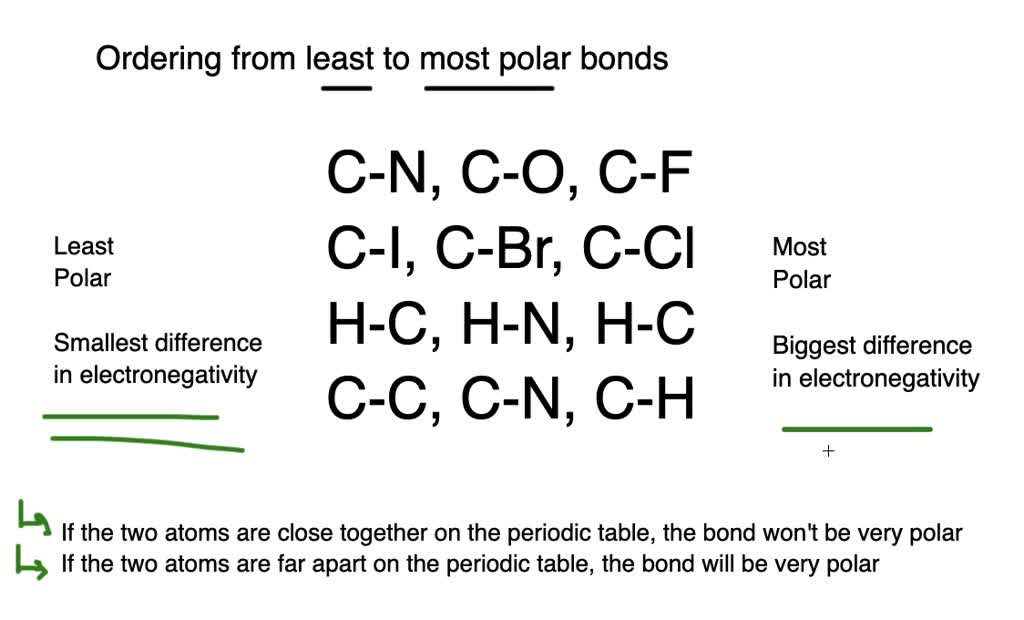
Nitrogen is more electronegative than carbon which is more electronegative than hydrogen. Electronegativity difference must be less than 0.5. Which of the following bonds would be the least polar? Which of the following bonds is least polar? Sindh mcqs, 11th class mcqs, chemistry mcqs, chemical bonding mcqs, h—f , o—o , c—c , all of these Polar bond forms when electrons are unequally shared by two atoms.
 Source: chegg.com
Source: chegg.com
That would be carbon and iodine. The electronegativity difference between the two atoms will be calculated. Carbon�s electronegativity is 2.6 and oxygen�s electronegativity is 3.4.
![Solved:rank The Following Bonds From Highest Polarity To The Lowest: 1-Most Polar 4 = Least Polar N–Ci [Select ] C-Si [Select ] O–P [Select ] A-C [Select ] Solved:rank The Following Bonds From Highest Polarity To The Lowest: 1-Most Polar 4 = Least Polar N–Ci [Select ] C-Si [Select ] O–P [Select ] A-C [Select ]](https://cdn.numerade.com/ask_images/38b14a43612c484fa0eaed562246b603.jpg) Source: numerade.com
Source: numerade.com
N is about 3.0 and h is 2.1 so the different is about 0.9; In which case is the bond polarity incorrect? Bond polarity can be calculated, examining the pauling scale electronegativity values of the two atoms.

(the difference in the electronegativies of the two atom is least) Polar covalent bonds can be present in a nonpolar molecule. Covalent compounds are soluble in.
 Source: chegg.com
Source: chegg.com
In which case is the bond polarity incorrect? (the difference in the electronegativies of the two atom is least) Which of the following covalent bonds is the least polar?
Which of the following bonds is the least polar if the pauling electronegativity scale is used to gauge the polarity of bonds? Polar covalent bond is present if the electronegativity difference. The electronegativity decreases from left to right;
 Source: oneclass.com
Source: oneclass.com
Which of the following bonds is least polar? A) rb b) ga c) n d) ar e) i 10. Which of the following bonds would be the least polar?
 Source: slideplayer.com
Source: slideplayer.com
That would be carbon and iodine. There will always be a small amount of electron sharing. Between atoms is equal or less than 0.4.
 Source: slidetodoc.com
Source: slidetodoc.com
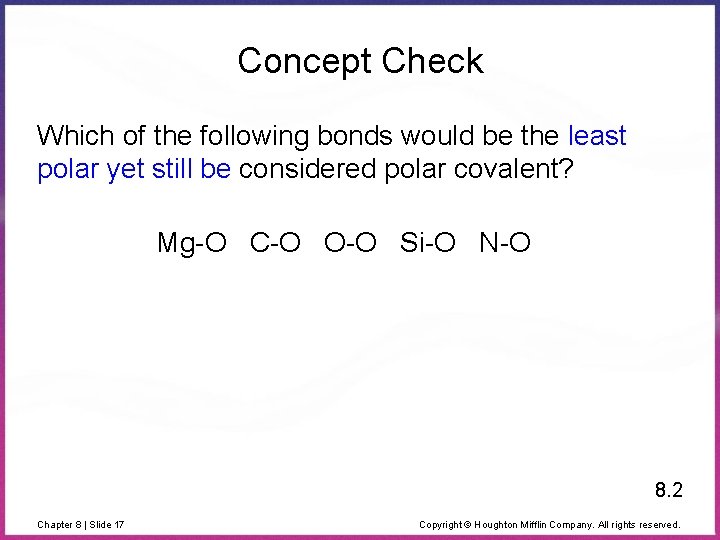
From the calculated en difference, the least polar is the bond between hydrogen and boron, a. The electronegativity difference between the two atoms will be calculated. The least polar bond would be between atoms have have the smallest difference in electronegativity.
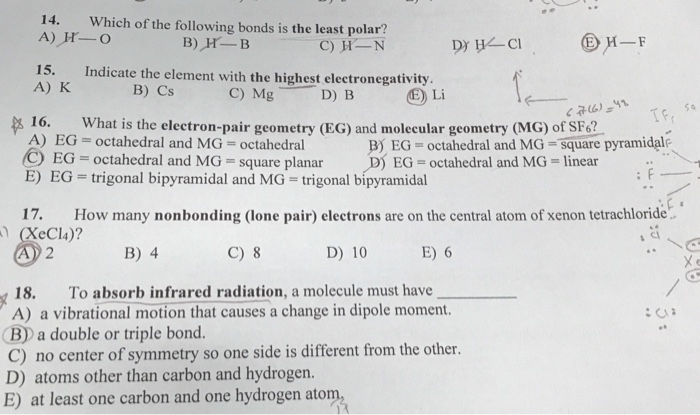
Sindh mcqs, 11th class mcqs, chemistry mcqs, chemical bonding mcqs, h—f , o—o , c—c , all of these We�ve got l i b r, followed by clc out, followed by casey l. Polar covalent bond is present if the electronegativity difference.
 Source: chegg.com
Source: chegg.com
For the elements cs, f, and p, the. Recall that for a covalent bond to be: A) co b) hf c) scl d).
 Source: slideplayer.com
Source: slideplayer.com
Which of the following bonds is least polar? For the elements cs, f, and p, the. Which of the following covalent bonds can be classified as being the least polar?
 Source: bartleby.com
Source: bartleby.com
Here is the trend of electronegativity difference (∆en) that also shows the bond polarity of the molecules.c to c < c to n < c to cl < c to o < c to fl. Which of the following bonds would be the least polar? Between atoms is equal or less than 0.4.
 Source: numerade.com
Source: numerade.com
Electronegativity difference must be less than 0.5. They will be the two elements which have electronegativities that are closest. Which of the following bonds is the least polar if the pauling electronegativity scale is used to gauge the polarity of bonds?
 Source: chegg.com
Source: chegg.com
N is about 3.0 and h is 2.1 so the different is about 0.9; Where q the magnitude of the charge on each end of the dipole and r is the distance between the. So the bigger difference in election negativity between the two atoms are bonded means that the bond is more polarized.
 Source: chegg.com
Source: chegg.com
Nitrogen is more electronegative than carbon which is more electronegative than hydrogen. Here is the trend of electronegativity difference (∆en) that also shows the bond polarity of the molecules.c to c < c to n < c to cl < c to o < c to fl. That would be carbon and iodine.
 Source: chegg.com
Source: chegg.com
Electronegativity difference must be less than 0.5. Which of the following bonds is polar?? Carbon�s electronegativity is 2.6 and oxygen�s electronegativity is 3.4.
 Source: clutchprep.com
Source: clutchprep.com
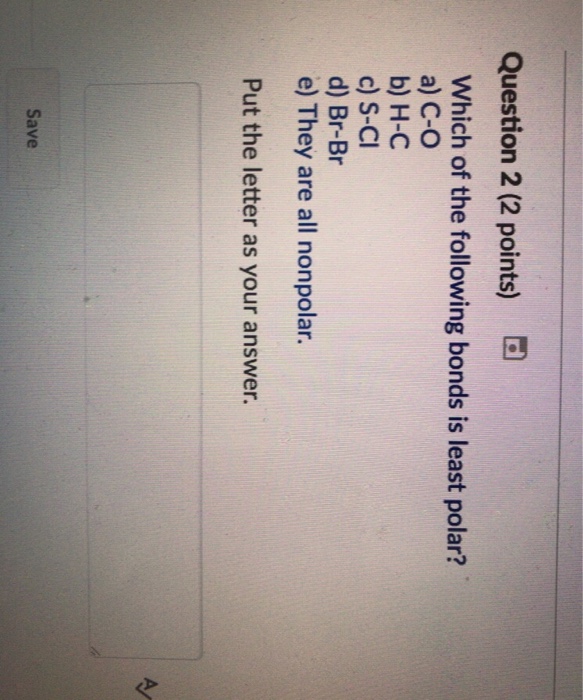
A) c—o b) h—c c) s—cl d) br—br e) they are all nonpolar. 100% (10 ratings) 1.the least polar bond will have the lowest electronegativity differece. (the difference in the electronegativies of the two atom is least)
 Source: chegg.com
Source: chegg.com
This higher the difference, the more polar the bond is. So the bigger difference in election negativity between the two atoms are bonded means that the bond is more polarized. Electronegativity can defined as the ability of an atom to attract the bonding electron pair towards itself.this result in the generation of polarity.
 Source: youtube.com
Source: youtube.com
N is about 3.0 and h is 2.1 so the different is about 0.9; Nitrogen is more electronegative than carbon which is more electronegative than hydrogen. In practice no bond is totally ionic.
 Source: chegg.com
Source: chegg.com
We�ve got l i b r, followed by clc out, followed by casey l. Using your periodic table, you can locate each element and see the distance between the two and, generally, the further apart they are, the more polar the bond will be. Polar covalent bonds can be present in a nonpolar molecule.
Also Read :