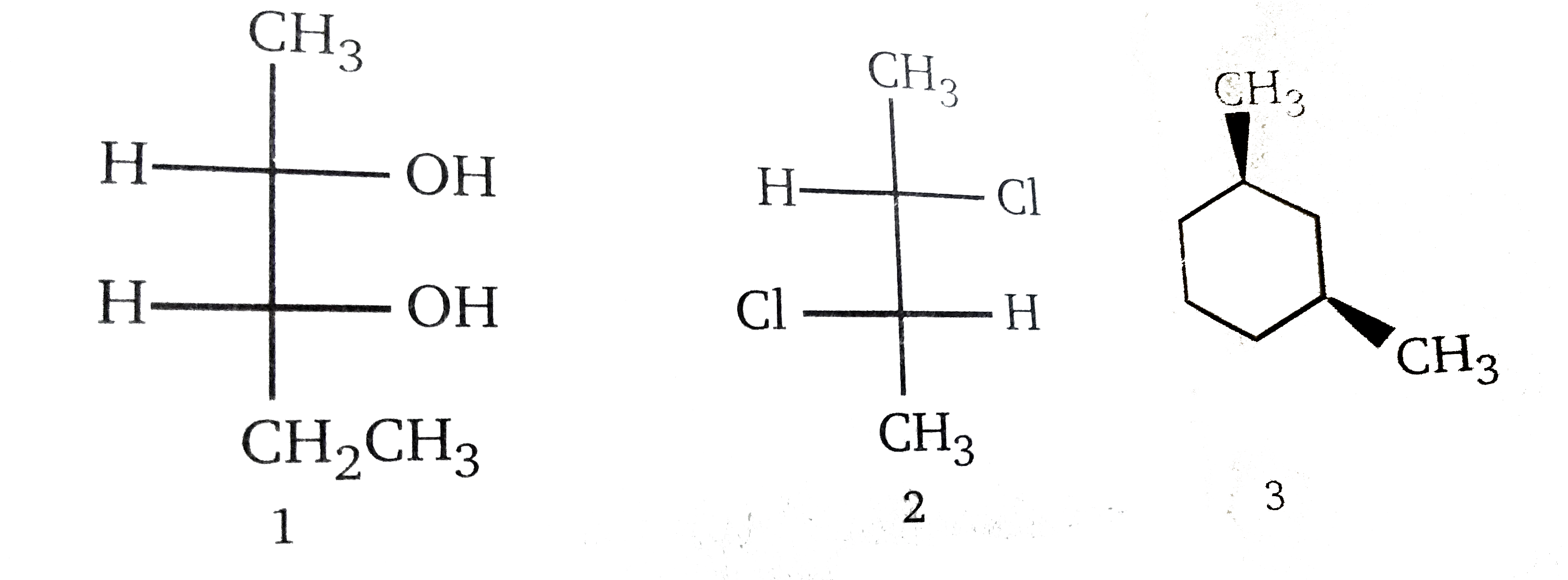
Racemic mixture as well as meso compounds are optically inactive. Which of the following is are meso compounds h ch 3 ch 3 cl cl h cl cl h ch 3 ch from chm 2210 at florida international university
Which Of The Following Are Meso Compounds. In short, the enantiomer of this compound is the compound itself, and this compound is not meso. Which of the following is a meso compound? Chiral molecules are represented by: Meso compounds that contain chirality centers, but possess a plane of symmetry.
 Which Of The Following Compounds Are Meso Forms ? <Img Src="Https://D10Lpgp6Xz60Nq.cloudfront.net/Physics_Images/Msc_Org_Chm_C02_E01_033_Q01.Png" Width="80%"> From doubtnut.com
Which Of The Following Compounds Are Meso Forms ? <Img Src="Https://D10Lpgp6Xz60Nq.cloudfront.net/Physics_Images/Msc_Org_Chm_C02_E01_033_Q01.Png" Width="80%"> From doubtnut.com
Related Post Which Of The Following Compounds Are Meso Forms ? <Img Src="Https://D10Lpgp6Xz60Nq.cloudfront.net/Physics_Images/Msc_Org_Chm_C02_E01_033_Q01.Png" Width="80%"> :
The reaction is an example of. Thus our answer will be option d. In this side it is cl in this side it is edge. Ch3 h3c oh ho 8) label each asymmetric carbon in the compound below as r or s.
A meso compound contains chiral centers but is superimposable on its mirror image, rendering it achiral and optically inactive.
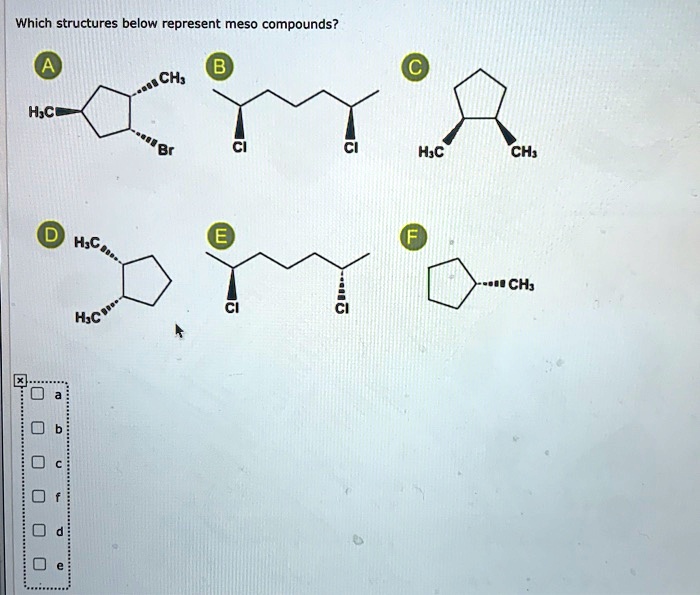
Asked apr 27, 2019 in chemistry by bhawna (68.4k points) upsee; Enantiomers, diastereomers, or the same compound? Which, if any, of the following structures represent meso compounds? One pair of enantiomers (a and b) and an achiral molecule c, called a “meso compound.” a meso compound is an achiral molecule that nonetheless contains a stereogenic atom. A meso compound contains chiral centers but is superimposable on its mirror image, rendering it achiral and optically inactive. In this side it is cl in this side it is edge.
 Source: oneclass.com
Source: oneclass.com
It will rotate plane polarized light. It is atomically symmetrical → symmetrical in connectivity. A) the specific rotation is 0°.

A meso compound generally has a plane of symmetry. In this side it is shell and in this side it�d edge. (a) 1 (b) 2 (c) 3 (d) 4.
 Source: byjus.com
Source: byjus.com
The third choice can and cannot exist as a meso compound, but b/c of the type of the question, i�d say 3 can exist as a meso compound. The reaction is an example of. Yes, meso compounds do have chiral centers but they are all inverted.
 Source: numerade.com
Source: numerade.com
It will rotate plane polarized light. In this problem, this compound, which i am writing here, just look at it carefully. So looking at this first material, typically what you could do with a musical compound is look for a plane of symmetry.
 Source: toppr.com
Source: toppr.com
The reaction is an example of. Ch3 h3c oh ho 8) label each asymmetric carbon in the compound below as r or s. Stereoisomers are defined as the isomers that differ in the spatial arrangement of the atoms in a co.
 Source: toppr.com
Source: toppr.com
(a) (b) (c) (d) (e) (f) (g) (h) hexane, ch3 (ch2)4ch3, or nonane, ch3 (ch2)7ch3. Oh ch3 9) label each asymmetric carbon in the compound below as r or s. A compound will be meso if it follows these criteria:
 Source: oneclass.com
Source: oneclass.com
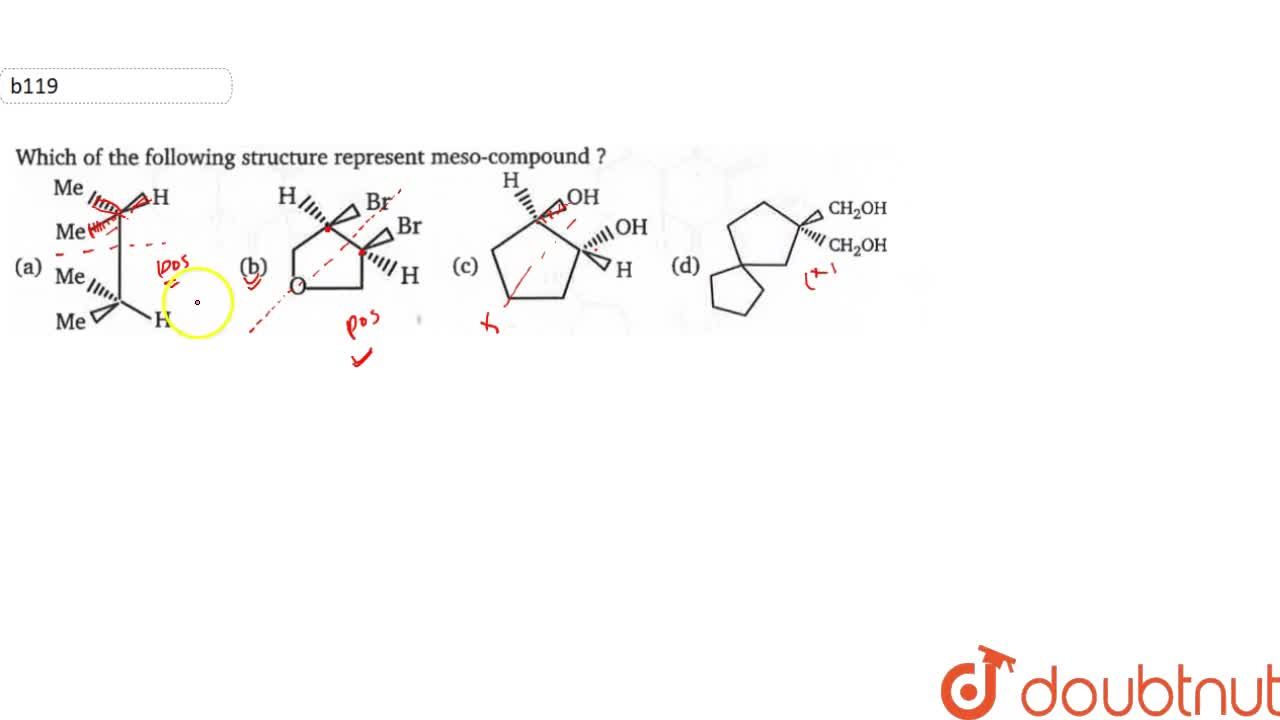
Stereoisomers are defined as the isomers that differ in the spatial arrangement of the atoms in a co. In the given compounds, only structure (b) has plane of symmetry, hence it is a meso compound. Meso compounds have an internal plane of symmetry, and their chiral centers have opposite r&s configurations.
 Source: doubtnut.com
Source: doubtnut.com
A compound will be meso if it follows these criteria: In option a,b, and c there are two stereocenters but because of a plane of symmetry, they are not optically active. Which of the following is not true for a meso compound:
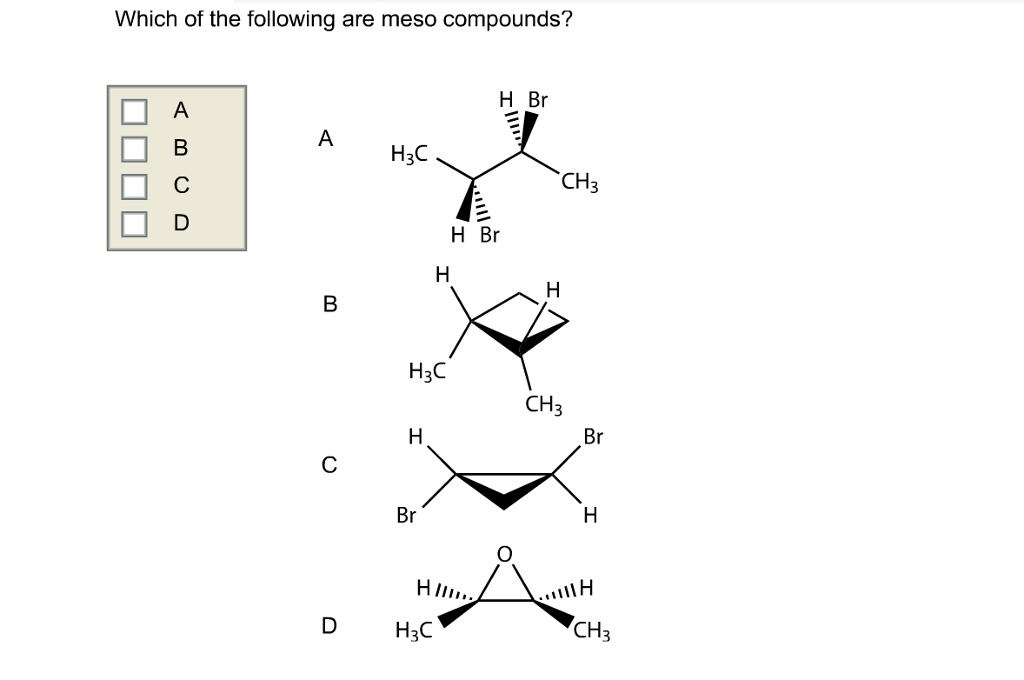 Source: chegg.com
Source: chegg.com
Which, if any, of the following structures represent meso compounds? So according to the option option c. The third choice can and cannot exist as a meso compound, but b/c of the type of the question, i�d say 3 can exist as a meso compound.
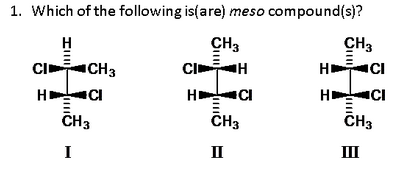 Source: chegg.com
Source: chegg.com
In the given compounds, only structure (b) has plane of symmetry, hence it is a meso compound. A meso compound is achiral. In this side it is cl in this side it is edge.
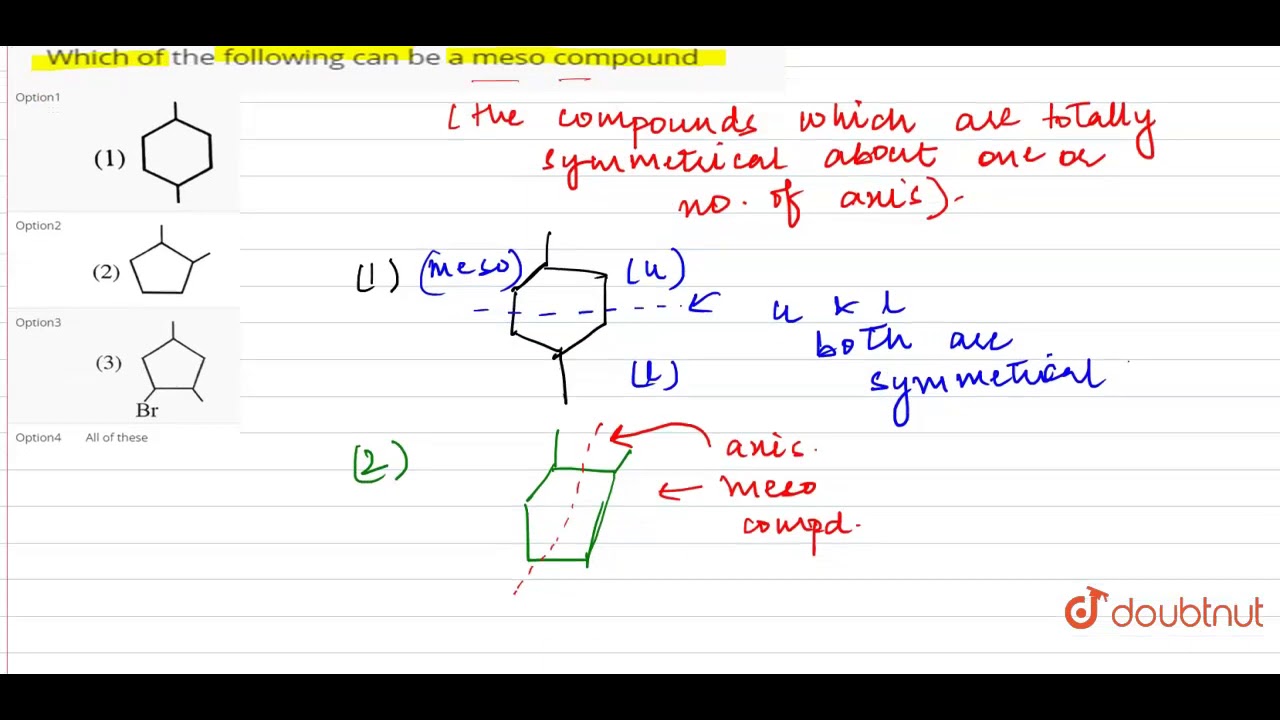 Source: youtube.com
Source: youtube.com
A meso compound is superimposable on its mirror image. All the four compounds (a), (b), (c) and (d) contain above mentioned features. A meso compound and its mirror image are identical.
 Source: doubtnut.com
Source: doubtnut.com
A common strategy used to distinguish the. The question asks which molecule can exist as a meso compound. Stereoisomers are defined as the isomers that differ in the spatial arrangement of the atoms in a co.
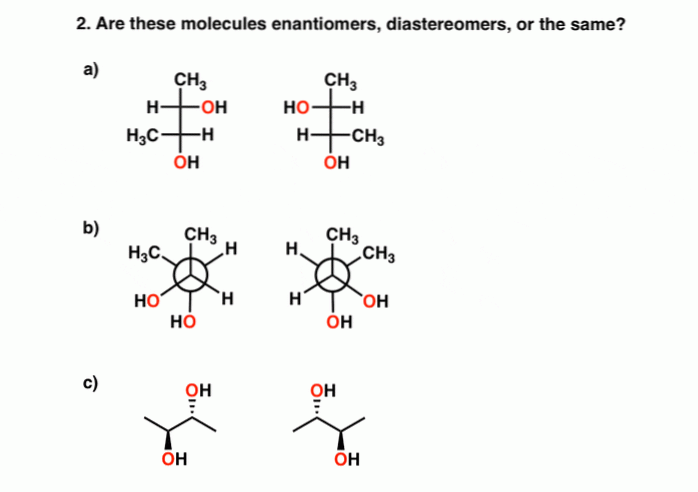 Source: en.differbetween.com
Source: en.differbetween.com
Every r is inverted to s and every s is inverted into r: Which, if any, of the following structures represent meso compounds? 1) meso compounds are achiral.
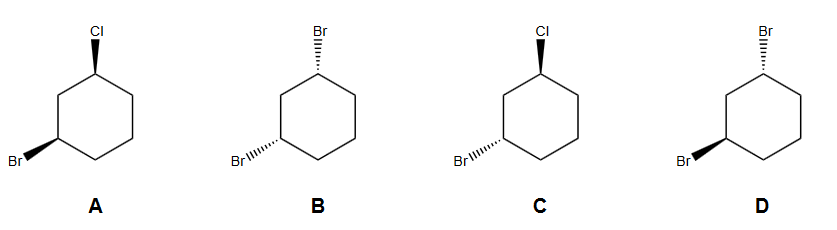 Source: chegg.com
Source: chegg.com
(a) only 1 (b) only 1 and 2 (c) only 2 and 3 (d) 1, 2 and 3. Which of the following compounds is/are chiral? 1) meso compounds are achiral.
 Source: ochempal.org
Source: ochempal.org
Asked apr 27, 2019 in chemistry by bhawna (68.4k points) upsee; A meso compound generally has a plane of symmetry. The structures that have plane of symmetry, which can divide it into two equal and identical halves are called meso structures.
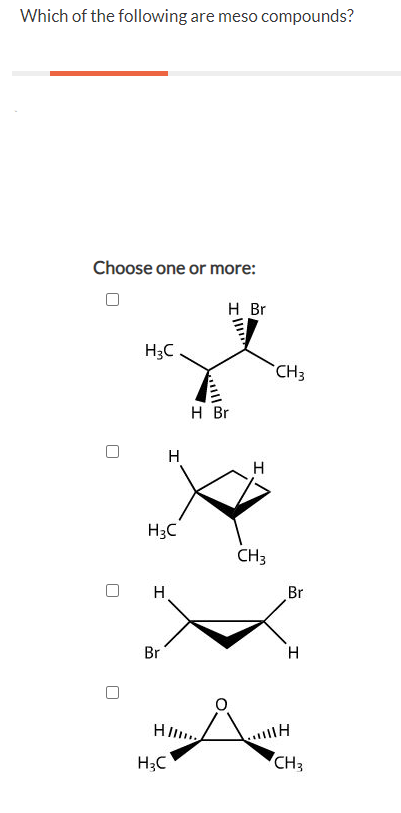 Source: chegg.com
Source: chegg.com
Which of the following is a meso compound? Which of the following is are meso compounds h ch 3 ch 3 cl cl h cl cl h ch 3 ch from chm 2210 at florida international university A meso compound generally has a plane of symmetry.
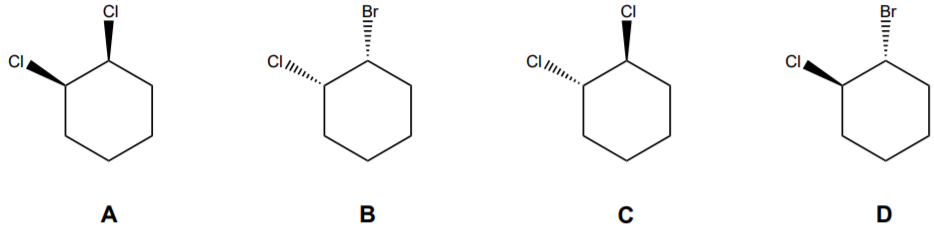 Source: chegg.com
Source: chegg.com
Asked apr 27, 2019 in chemistry by bhawna (68.4k points) upsee; Every r is inverted to s and every s is inverted into r: And if that symmetry exists through the compound, then there it must be music compound the neighbors always going to be exactly the same.
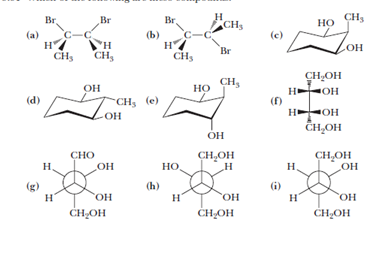 Source: chegg.com
Source: chegg.com
Meso compounds are non optically active member of a set of stereoisomers despite having stereogenic centres the molecule is not chiral. The question asks which molecule can exist as a meso compound. Which of the following statements about a meso compound is false?
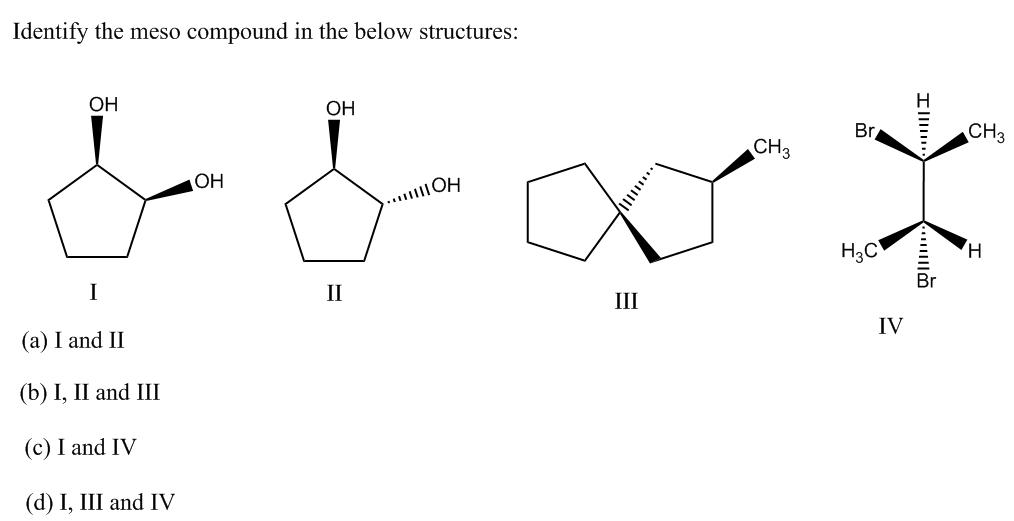 Source: bartleby.com
Source: bartleby.com
A) the specific rotation is 0°. Racemic mixture as well as meso compounds are optically inactive. A common strategy used to distinguish the.
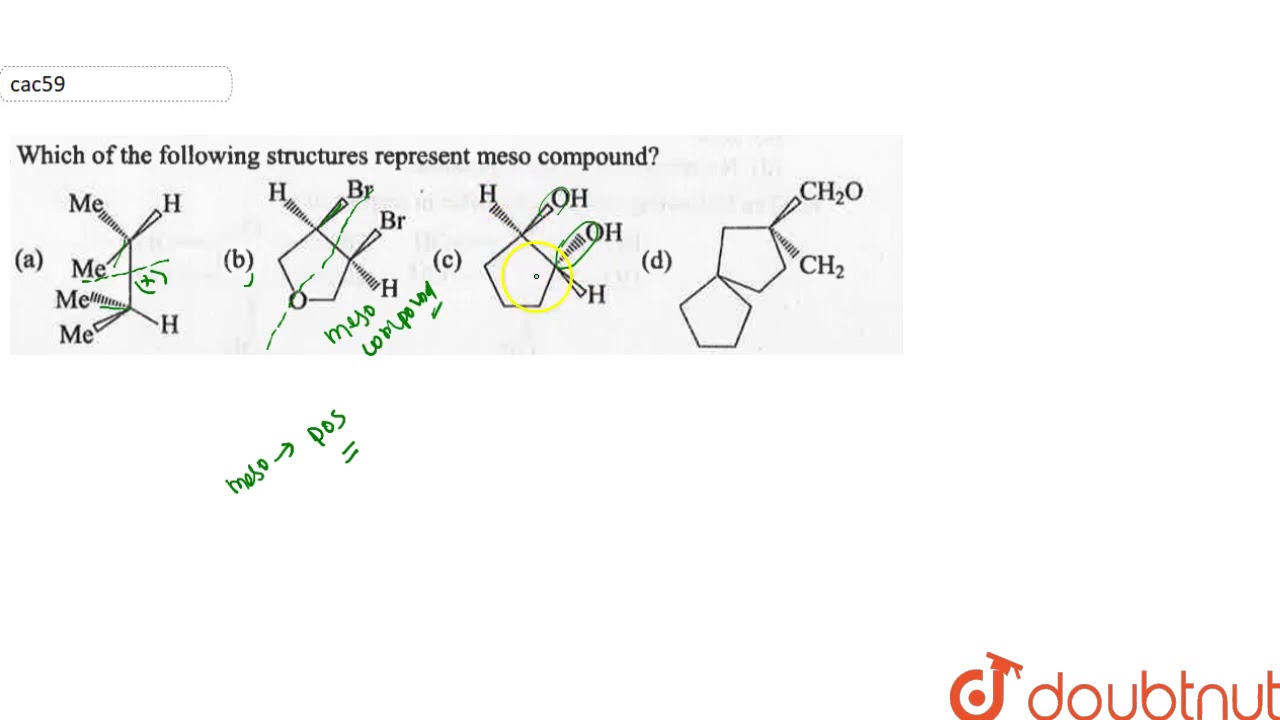 Source: youtube.com
Source: youtube.com
A common strategy used to distinguish the. 1) meso compounds are achiral. Thus our answer will be option d.
Also Read :