Zero ( 0.0) pure covalent. In other words, they have 1,1 relationships.
Which Of The Following Are Compounds. So, the correct answer is option d. Which of the following compounds have conjugated double bonds? Asked jul 16, 2020 in chemistry by hitheshkumar (85.2k points) which of the following compounds has the largest dipole moment ? Good i ii iii iv 3.
 Solved Which Of The Following Are Elements? Which Are | Chegg.com From chegg.com
Solved Which Of The Following Are Elements? Which Are | Chegg.com From chegg.com
Related Post Solved Which Of The Following Are Elements? Which Are | Chegg.com :
Predict the product of the following reaction. Tautomerism is a functional isomerism wherein the two structures which are isomers readily interconvert to each other and exist in dynamic equilibrium with each other. A) ch 4 b) so 2 c) cacl 2 d) co 2 e) h 2 o 2. In this organometallic compound,bond between cobalt and co ligands have both σ and π character.
The reaction of wcl6 with al at 400 c gives black crystal s of a compound containing only tungsten and chlorine.
Which of the following compounds will have the highest molar solubility in pure water? Which of the following compounds gives a secondary alcohol upon reaction with methylmagnesium bromide? Which of the following compounds have conjugated double bonds? C f 4 c f 4. A) ch 4 b) so 2 c) cacl 2 d) co 2 e) h 2 o 2. C h 3f c h 3 f.
 Source: chegg.com
Source: chegg.com
C h 3f c h 3 f. Which of the following compounds is likely to occur as a solid at room temperature? A compound was found to be soluble in water.
 Source: youtube.com
Source: youtube.com
Which of the following compounds is likely to occur as a solid at room temperature? C h 3c h 2c h 2oh c h 3 c h 2 c h 2 o h. Which of the following taxonomical ranks contain organisms least similar to one another.
 Source: youtube.com
Source: youtube.com
Which of the following compounds, when heated at 483 k turns to caramel? C h 3c h 2c h 2oh c h 3 c h 2 c h 2 o h. Aromatic compounds are categorized further into two categories i.e.
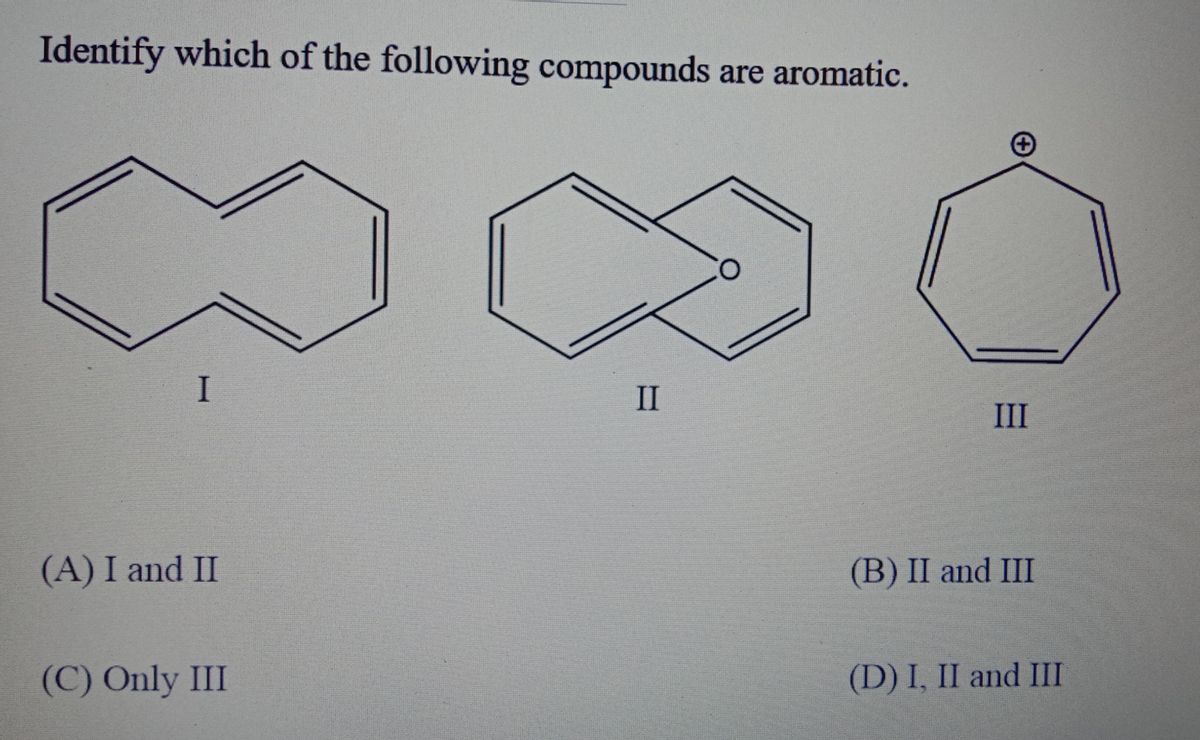 Source: bartleby.com
Source: bartleby.com
Tautomerism is a functional isomerism wherein the two structures which are isomers readily interconvert to each other and exist in dynamic equilibrium with each other. C h 3f c h 3 f. In this organometallic compound,bond between cobalt and co ligands have both σ and π character.
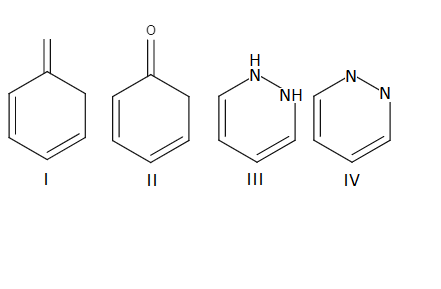 Source: study.com
Source: study.com
C h 4 c h 4. C h 4 c h 4. For a reaction between gaseous compounds, $\hspace20mm 2a + b \longrightarrow c + d$ the reaction rate = k[a][b].
 Source: chegg.com
Source: chegg.com
The density of glycerol is higher than propanol due to. Which of the following pairs of compounds is isoelectronic and isostructural? Which one of the following cations would form a precipitate when added to an aqueous solution of this compound?
 Source: slideplayer.com
Source: slideplayer.com
Asked jul 16, 2020 in chemistry by hitheshkumar (85.2k points) which of the following compounds has the largest dipole moment ? Aromatic compounds are categorized further into two categories i.e. Bec l2, xef 2 b e c l 2, x e f 2.
 Source: clutchprep.com
Source: clutchprep.com
A) ph 3 b) no 2 c) mgcl 2 d) so 2 e) clo 2. In fact, magnesium chloride has a greater degree of covalency as compared to sodium chloride. C h 3c ooh c h 3 c o o h.
 Source: toppr.com
Source: toppr.com
A sample of this compound, when reduced with hydrogen, gives 0.2232 g of tungsten metal and hydrogen chloride, which is absorbed in water. The reaction of wcl6 with al at 400 c gives black crystal s of a compound containing only tungsten and chlorine. (since the algebraic sum of the oxidation number of all atoms in a compound must be zero).
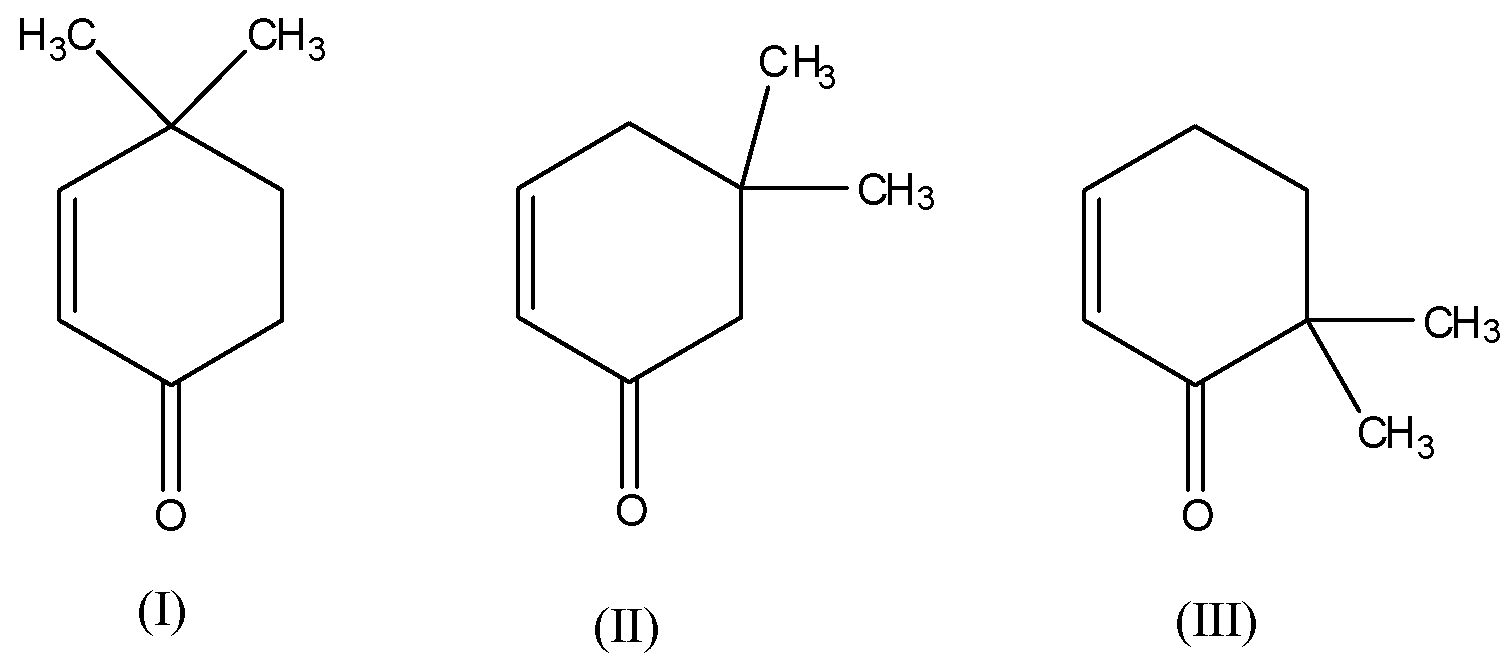 Source: vedantu.com
Source: vedantu.com
In other words, they have 1,1 relationships. Aromatic compounds are categorized further into two categories i.e. Which of the following compounds have conjugated double bonds?
 Source: clutchprep.com
Source: clutchprep.com
So, we can calculate the oxidation state of chlorine by this method : Which of the following compounds have isolated double bonds? Predict the product of the following reaction.
 Source: clutchprep.com
Source: clutchprep.com
Which of the following taxonomical ranks contain organisms least similar to one another. Geminal or gem dihalides are those compounds in which halides are substituted on the same carbon atom. Which of the following compounds have isolated double bonds?

Amongest the following maximum number of compounds which give iodoform test are ch_(3)conh_(2),ch_(3)cocl, chcooh,(ch_(3)co)_(2) c_(6)h_(5)coch_(3),c_(6)h. Which of the following compounds will have the highest molar. C h 4 c h 4.
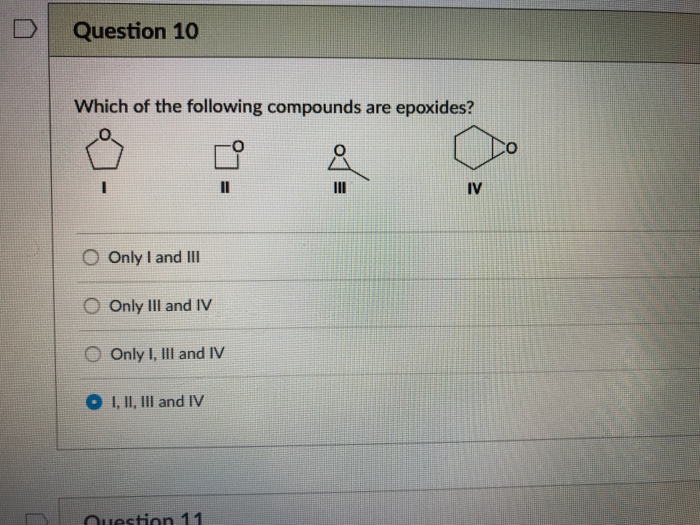
It was also found that addition of acid to an aqueous solution of this compound resulted in the formation of carbon dioxide. (a) chlorine gas (b) potassium chloride (c) iron (d)iron sulphide (e) aluminium (f) iodine (g) carbon (h) carbon monoxide (i) sulphur powder. A sample of this compound, when reduced with hydrogen, gives 0.2232 g of tungsten metal and hydrogen chloride, which is absorbed in water.
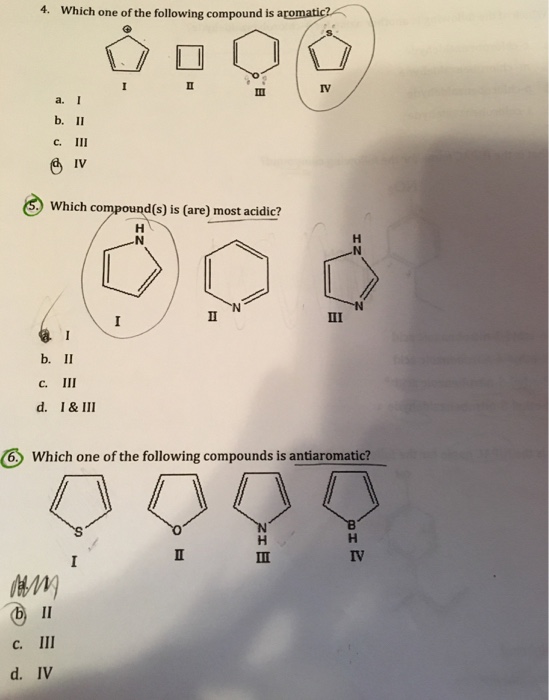
In other words, they have 1,1 relationships. Which one of the following compounds will react with two mole of c h 3m gbr c h 3 m g b r ? Which of the following are not compounds?
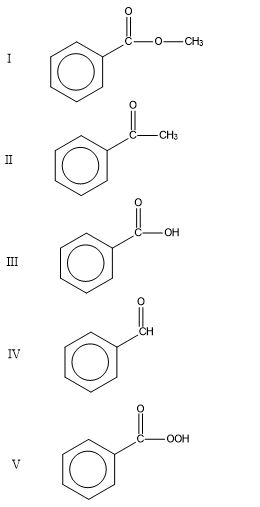 Source: study.com
Source: study.com
Which of the following compounds is likely to occur as a solid at room temperature? Closed nov 30, 2021 by kumari prachi. In which of the following compounds, the oxidation number of iodine is fractional ?
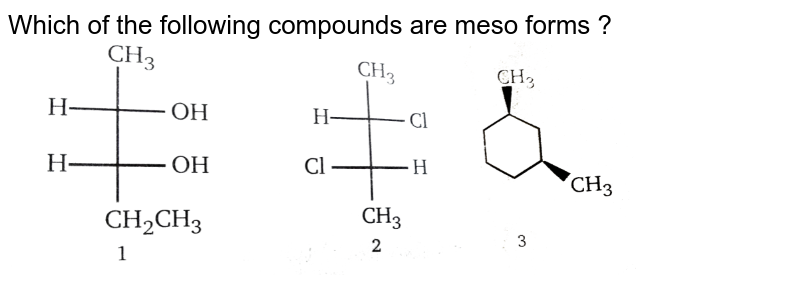 Source: doubtnut.com
Source: doubtnut.com
Which of the following compounds are isolated dienes? (since the algebraic sum of the oxidation number of all atoms in a compound must be zero). Also, magnesium is more electronegative as compared to sodium.
 Source: toppr.com
Source: toppr.com
Tautomerism is a functional isomerism wherein the two structures which are isomers readily interconvert to each other and exist in dynamic equilibrium with each other. Good i ii iii iv 3. 85% (252 ratings) sign up for free to view this solution.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Sodium chloride (nacl) has a melting point of 801 degree celsius, while that of magnesium chloride (mgcl2) is 714 degrees celsius. Which of the following are not compounds? Which of the following taxonomical ranks contain organisms least similar to one another.
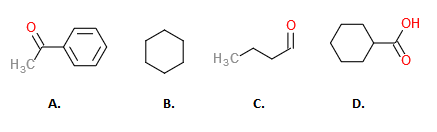 Source: study.com
Source: study.com
So, the correct answer is option d. The reaction of wcl6 with al at 400 c gives black crystal s of a compound containing only tungsten and chlorine. Predict the product of the following reaction.
Also Read :





