Hence, hexane (c6h14) has the maximum boiling point. With the same number of carbons) the alkane should have a higher boiling point.
Which Of The Following Alkanes Has The Highest Boiling Point. Ethanol contains oxygen and hydrogen can form hydrogen bonds which require more energy to be broken than the van der waals forces in ethanethiol. B) alkanes can form chains or cyclic structures. The alkenehexane has a boiling point of. D) alkanes are more dense than water.

Related Post Why Do Larger Alkanes Have Higher Boiling Points? - Quora :
Ethanol contains oxygen and hydrogen can form hydrogen bonds which require more energy to be broken than the van der waals forces in ethanethiol. As explained, since there is a bigger volume to an alkane than its corresponding alkyne (i.e. Each alkene has 2 fewer electrons than the alkane with the same number of carbons. Octane will have a higher boiling point than 2,2,3,3‑tetramethylbutane, because it branches less than 2,2,3,3‑tetramethylbutane, and therefore has a larger “surface area” and more van der waals forces.
Among isomeric alkanes, branching decreases boiling point.
In the case of melting point, alkanes containing even no. Of carbon atoms have high melting point than an odd number of carbon atoms. With branching surface area decreases and so boiling point also decreases. But, the forces in hexane will be stronger than those in butane because hexane molecules are larger and therefore capable of more extensive dispersion forces between each other than the molecules of butane. What is maximum boiling azeotrope? Hence the boiling point is low.
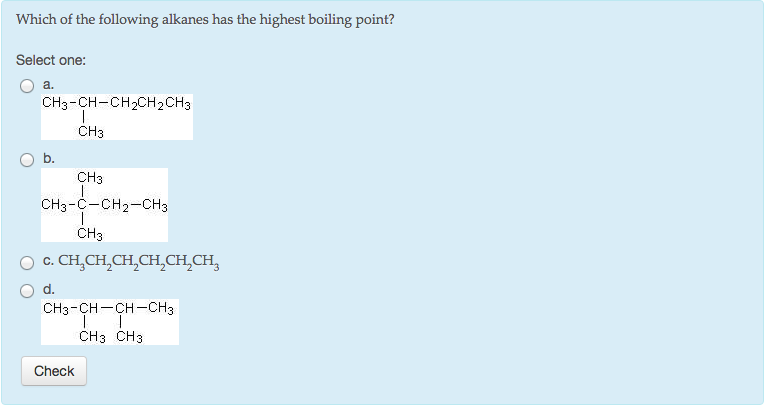 Source: chegg.com
Source: chegg.com
Which butane has the minimum boiling point? Explain why ethanol has the higher boiling points. A) alkanes are hydrocarbons that contain only single bonds.
 Source: chegg.com
Source: chegg.com
In the case of melting point, alkanes containing even no. However, there�s something else in play here: B) alkanes can form chains or cyclic structures.
 Source: brainly.in
Source: brainly.in
With the same number of carbons) the alkane should have a higher boiling point. Question 45 which of the following alkanes has the highest boiling point? But, the forces in hexane will be stronger than those in butane because hexane molecules are larger and therefore capable of more extensive dispersion forces between each other than the molecules of butane.
 Source: toppr.com
Source: toppr.com
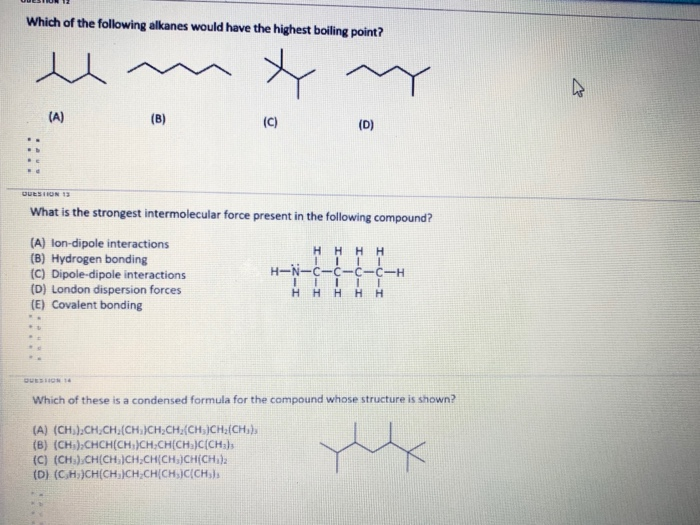
Boiling point of alkane increases with molar mass. Which of the following has maximum boiling point? C4h10 d.c6h14 e.c8h18 indicate which of the following molecules exhibits the greatest dispersion forces о а.ch4 ob.ch3ch2ch2ch3 с.
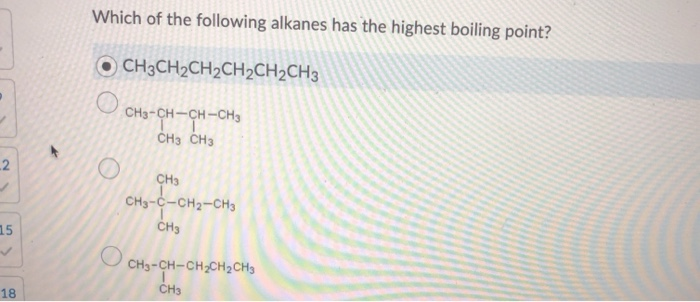 Source: chegg.com
Source: chegg.com
(2) ethylene glycol contains two carbon atoms and two hydroxyl. Among isomeric alkanes, branching decreases boiling point. In the case of melting point, alkanes containing even no.
 Source: formsbirds.com
Source: formsbirds.com
Closed sep 29 by urmillasahu. Maximum boiling azeotropes are those which have the boiling point higher than any of its constituents. As explained, since there is a bigger volume to an alkane than its corresponding alkyne (i.e.
 Source: numerade.com
Source: numerade.com
Which of the following straight chain alkanes has the highest boiling point? Which of the following alkanes will have the lowest biling point ? The alkenehexane has a boiling point of.
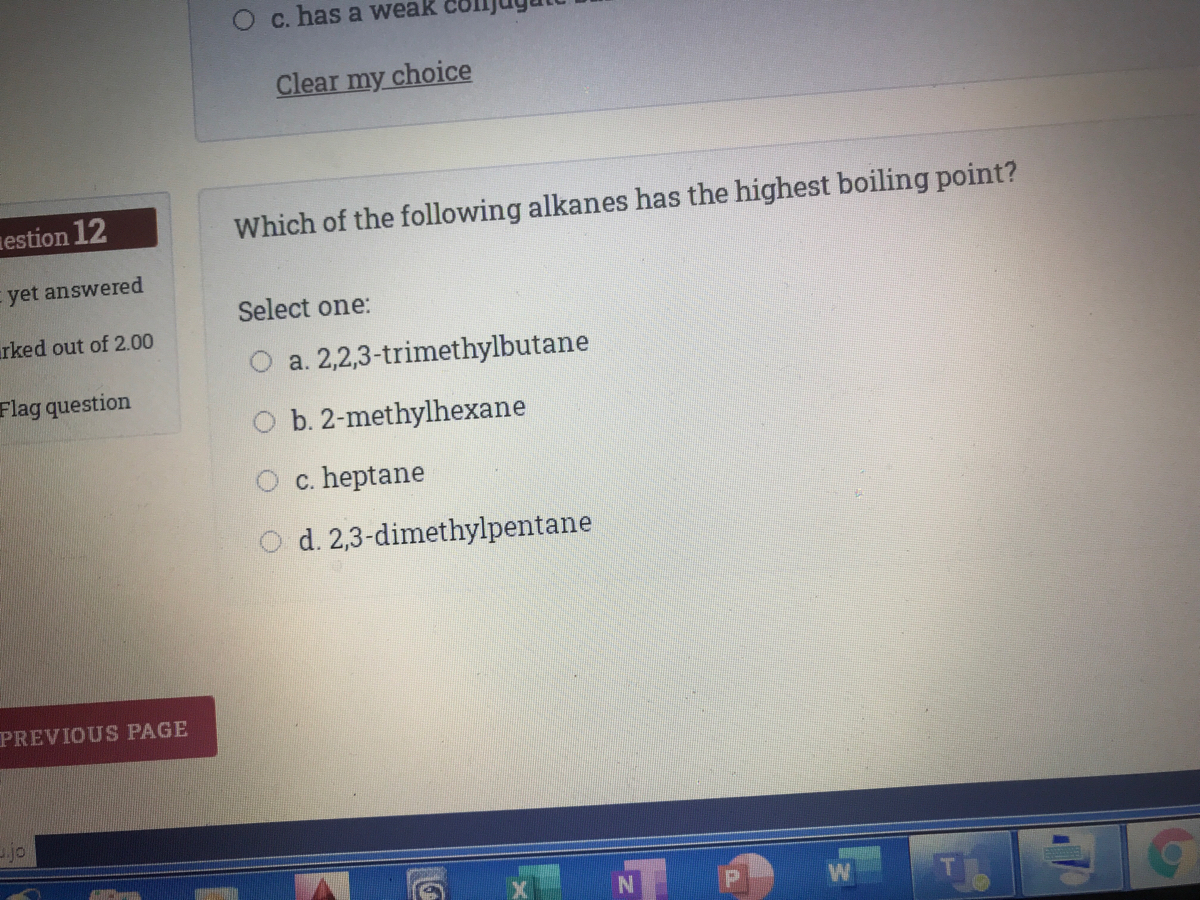 Source: bartleby.com
Source: bartleby.com
Which butane has the minimum boiling point? For this reason, hexane has a higher boiling point than butane. B) alkanes can form chains or cyclic structures.
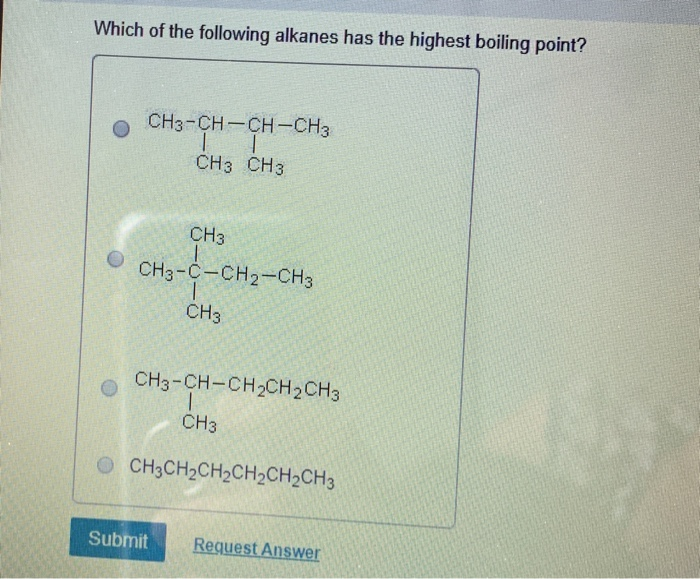 Source: chegg.com
Source: chegg.com
Closed sep 29 by urmillasahu. (2) ethylene glycol contains two carbon atoms and two hydroxyl. Which of the following alkanes will have the lowest biling point ?
 Source: askiitians.com
Source: askiitians.com
Of carbon atoms have high melting point than an odd number of carbon atoms.question: The alkenepentene has a boiling point of. The alkenehexane has a boiling point of.

Explain why the boiling point of a branched. Boiling point of alkane increases with molar mass. A) alkanes are hydrocarbons that contain only single bonds.

Boiling point of alkane increases with molar mass. The alkenehexane has a boiling point of. B) alkanes can form chains or cyclic structures.
 Source: toppr.com
Source: toppr.com
C4h10 d.c6h14 e.c8h18 indicate which of the following molecules exhibits the greatest dispersion forces о а.ch4 ob.ch3ch2ch2ch3 с. To give an indication of the variation in boiling points of different isomers of the same alkane, cycloalkanes have boiling points typically around 10 o c. Which of the following alkanes has the highest boiling point?
 Source: chegg.com
Source: chegg.com
Explain why ethanol has the higher boiling points. Which one of the following emissions from car exhausts is not classed as a pollutant? As explained, since there is a bigger volume to an alkane than its corresponding alkyne (i.e.
 Source: youtube.com
Source: youtube.com
With the same number of carbons) the alkane should have a higher boiling point. Maximum boiling azeotropes are those which have the boiling point higher than any of its constituents. Nonane will have a higher boiling point than octane, because it has a longer carbon chain than octane.
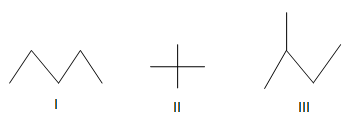 Source: study.com
Source: study.com
What is maximum boiling azeotrope? A) alkanes are hydrocarbons that contain only single bonds. Hence, hexane (c6h14) has the maximum boiling point.

However, there�s something else in play here: Why does hexane have a high boiling point? Which of the following straight chain alkanes has the highest boiling point?
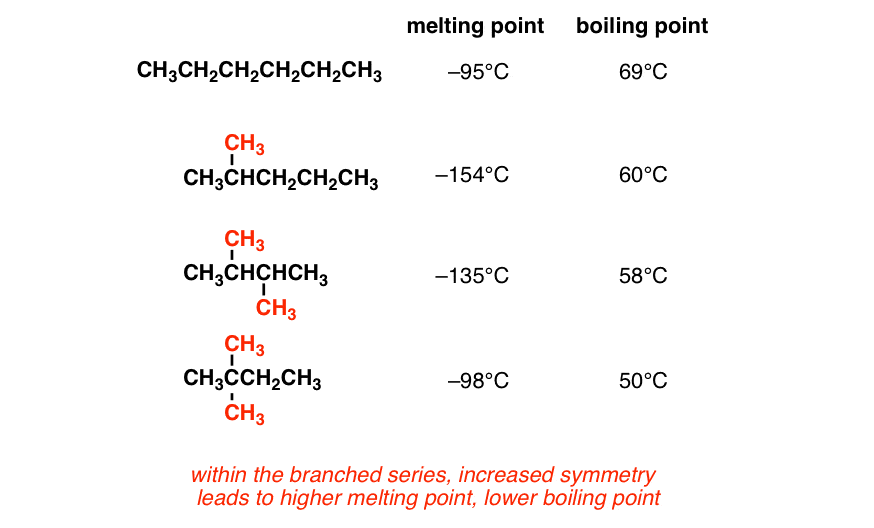 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
With the same number of carbons) the alkane should have a higher boiling point. A massless rod bd is suspended by two identical massless strings ab and cd of equal lengths. Hence, hexane (c6h14) has the maximum boiling point.
 Source: youtube.com
Source: youtube.com
But, the forces in hexane will be stronger than those in butane because hexane molecules are larger and therefore capable of more extensive dispersion forces between each other than the molecules of butane. Ethanol contains oxygen and hydrogen can form hydrogen bonds which require more energy to be broken than the van der waals forces in ethanethiol. However, there�s something else in play here:
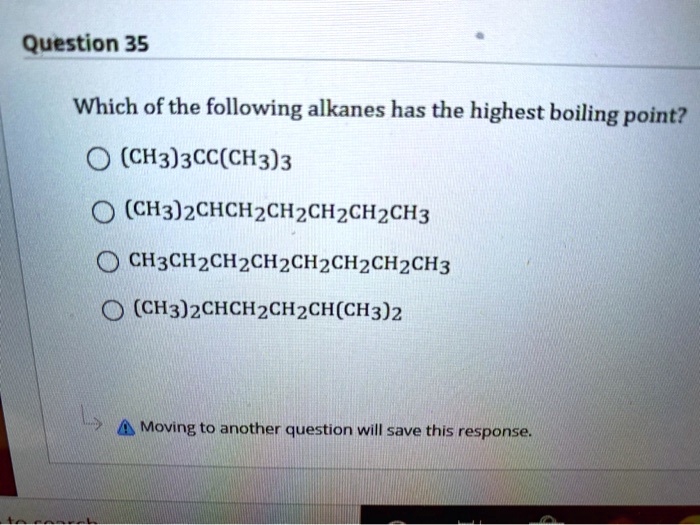 Source: numerade.com
Source: numerade.com
Which of the following compounds has the highest boiling point ?class:11subject: What is maximum boiling azeotrope? C) alkanes that contain only carbon and hydrogen atoms have low water solubility.
Also Read :