The weaker acid has the stronger conjugate base. Similarly, the weaker the acid, the stronger is its conjugate jugate base.
Which Of The Following Acids Has The Strongest Conjugate Base. Acetic acid (4.74) ammonium ion (9.3) lodic acid (0.77) nitrous acid (3.16) lactic acid (3.8) This makes its conjugate base cl(aq) a very weak base. So, we can say it is the strongest acid. Remember that the strongest conjugate base has a conjugate acid that is really weak.
 Answered: Which Of The Following Acids Has The… | Bartleby From bartleby.com
Answered: Which Of The Following Acids Has The… | Bartleby From bartleby.com
Related Post Answered: Which Of The Following Acids Has The… | Bartleby :
In the last we can conclude that the strongest acid is hclo$_4$. Acids and bases | acid and base strength key: The following acids are listed in order of decreasing acid. Hcl, hi, hbr, hno_3,h 2so4 and hclo_4#.
Acids are molecular covalent compounds which you don�t expect to ionize (release an h + and leave behind the conjugate base, or cl− for example).
If hcl is a strong acid, it must be a good proton donor. Which of the following acids will have the strongest conjugate base? Hcl is a strong acid. Which of the following weak acids has the strongest conjugate base? We can then make the connection that low pk a =strong acid= weak conjugate base. A weak acid shall produce a strong conjugate base.
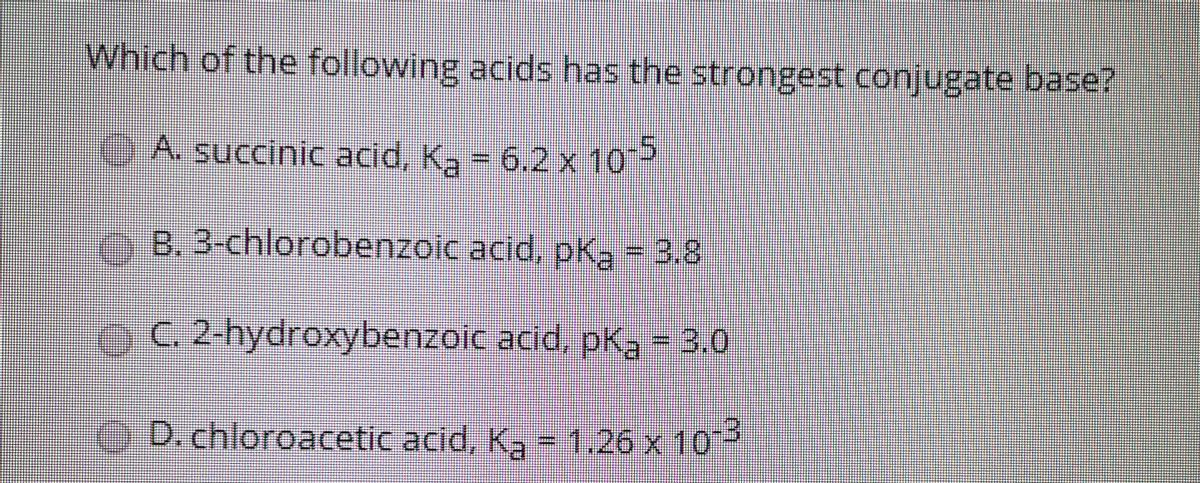 Source: bartleby.com
Source: bartleby.com
Acids and bases | acid and base strength key: D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. Acetic acid ($c{h_3}cooh$) and nitrous acid ($hn{o_2}$).
 Source: bartleby.com
Source: bartleby.com
The strongest acids ionize 100%. Reset help hci of the acids hcl or hf the one with the stronger conjugate base hf hso4 hzs of the acids hzpo4 or hno: Strong conjugate base has a weakconjugate acid.
 Source: doubtnut.com
Source: doubtnut.com
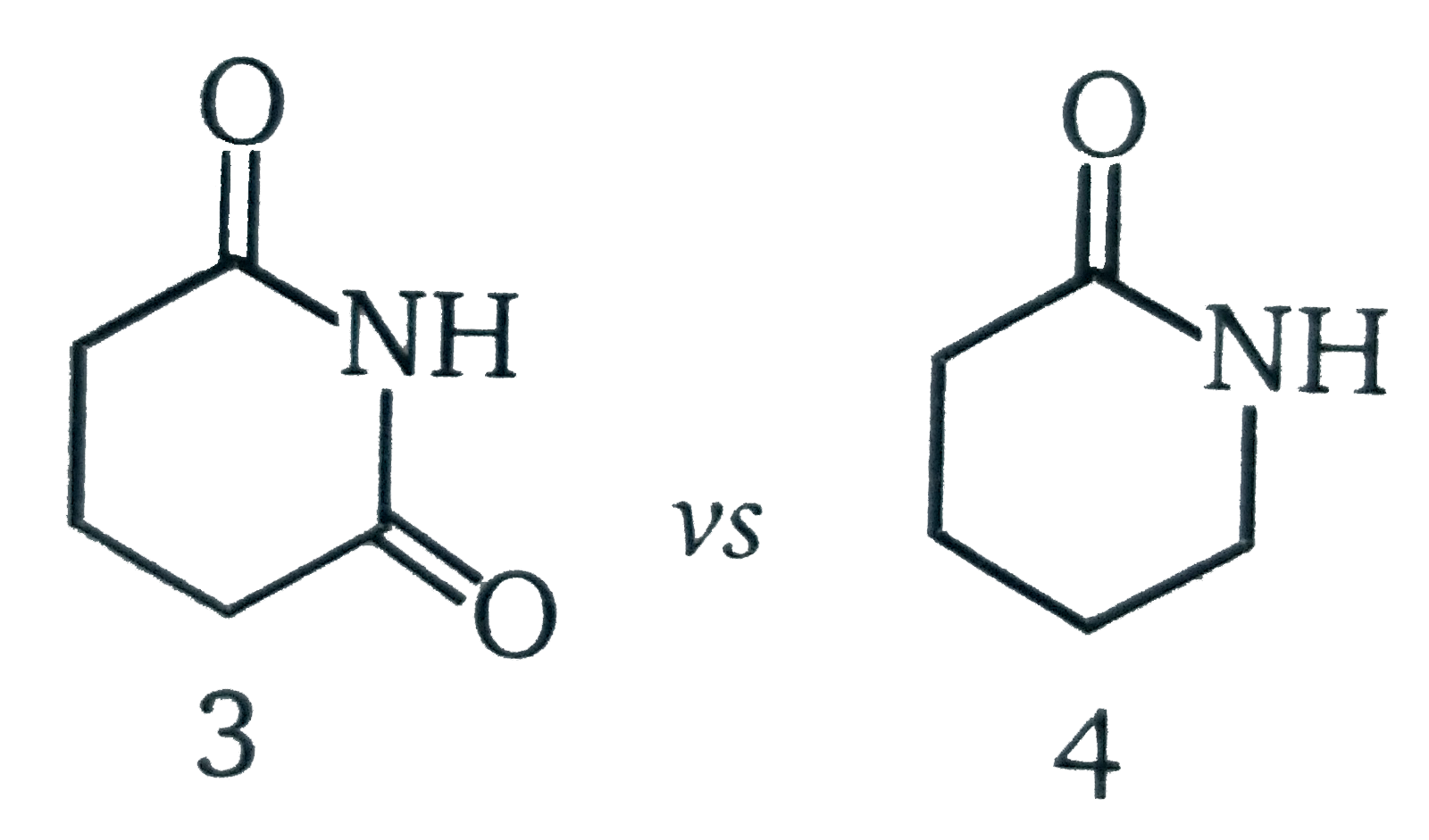
Here, the conjugate base is the weakest one, and we can say it is maximum stabilised. Here, the conjugate base is the weakest one, and we can say it is maximum stabilised. Acids with a smaller pk a are stronger than those with a high pk a.
 Source: clutchprep.com
Source: clutchprep.com
Strong acids have a weak conjugate base. The horizontal plane is present in one of the following molecules a. The h+ ion concentration in a solution prepared by mixing 50 ml of 0.20 m nacl , 25 ml of 0.10 m naoh and 25 ml of 0.30 m hcl will be a.
![Solved] Quiz Which One Of The Following Acids Has The Strongest Conjugate Base: A) Ascorbic Acid, Ka = 8.0 X 10�5 B) Benzoic Acid, Ka = 6.3 X 10�5 C… | Course Hero](https://www.coursehero.com/qa/attachment/3267230/ “Solved] Quiz Which One Of The Following Acids Has The Strongest Conjugate Base: A) Ascorbic Acid, Ka = 8.0 X 10�5 B) Benzoic Acid, Ka = 6.3 X 10�5 C… | Course Hero”) Source: coursehero.com
The following acids are listed in order of decreasing acid. The ethnic acid (acetic acid) is considered as a weak acid because it does notreleases all of its hydrogen in water; The stronger the acid, the weaker its conjugate base.
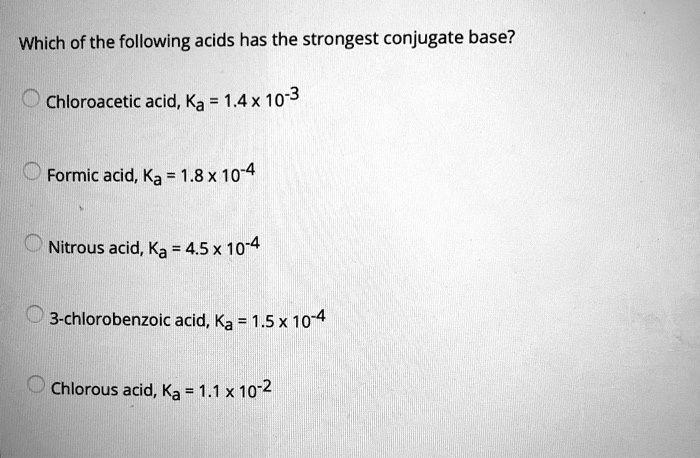 Source: itprospt.com
Source: itprospt.com
The conjugate acid of a. Which acid produces more ions? Rather it dissociates partially andestablishes equilibrium with its conjugate base.

D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. The following acids are listed in order of decreasing acid. The correct order of acidic strength is.

H f > h c ooh > h c n > h 2o h f > h c o o h > h c n > h 2 o. The h+ ion concentration in a solution prepared by mixing 50 ml of 0.20 m nacl , 25 ml of 0.10 m naoh and 25 ml of 0.30 m hcl will be a. Rather it dissociates partially andestablishes equilibrium with its conjugate base.
 Source: itprospt.com
Source: itprospt.com
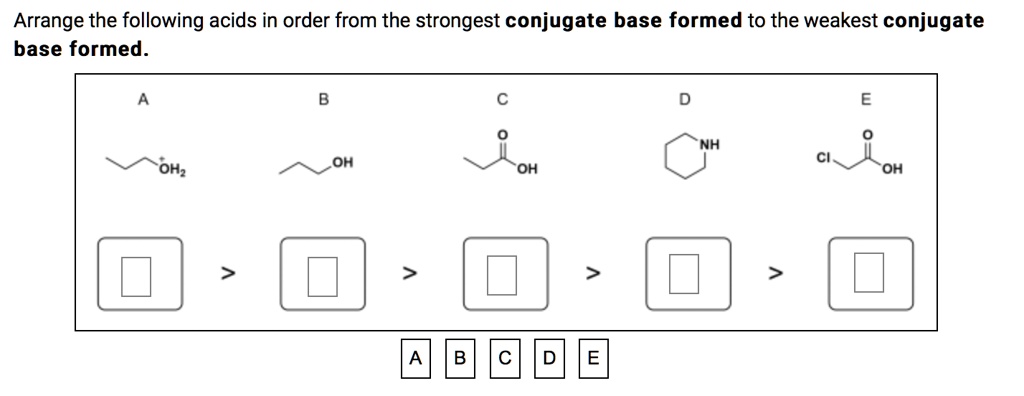
The general rules suggest that the stronger of a pair of acids must form the weaker of a pair of conjugate bases. Arrange the following acids in order from the strongest conjugate base formed to the weakest conjugate base formed. The acid strength goes up when the base strength goes down.) therefore, the strong acids that are listed have the weakest conjugate bases, removing responses a, d, and e.
 Source: slidetodoc.com
Source: slidetodoc.com
Rather it dissociates partially andestablishes equilibrium with its conjugate base. D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. Don’t get confused about why the conjugate base i.e.
 Source: numerade.com
Source: numerade.com
There are 6 that most consider to be the strong acids: The stronger the acid, the weaker its conjugate base. Here, the conjugate base is the weakest one, and we can say it is maximum stabilised.
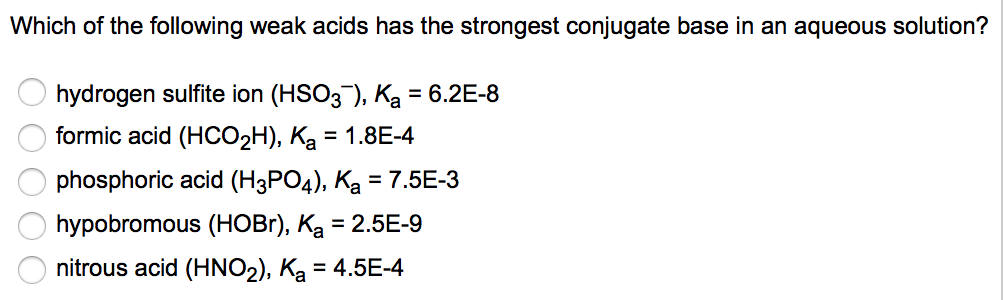 Source: chegg.com
Source: chegg.com
Acids with a smaller pk a are stronger than those with a high pk a. The pka value for each acid is given in parentheses. Nh oh2 oh 回、 回、 回、 回。 nh oh2 oh 回、.
 Source: oneclass.com
Source: oneclass.com
On the following page, there is a question that asks: Which of the following acids will have the strongest conjugate base? We can then make the connection that low pk a =strong acid= weak conjugate base.
 Source: chegg.com
Source: chegg.com
Their reasoning is that because perchloric acid is a strong acid, it will produce a. D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. Which of the following acids will have the strongest conjugate base?
 Source: chegg.com
Source: chegg.com
Acetic acid ($c{h_3}cooh$) and nitrous acid ($hn{o_2}$). Byjus asked on june 11, 2016 in chemistry. Arrange the following acids in order from the strongest conjugate base formed to the weakest conjugate base formed.
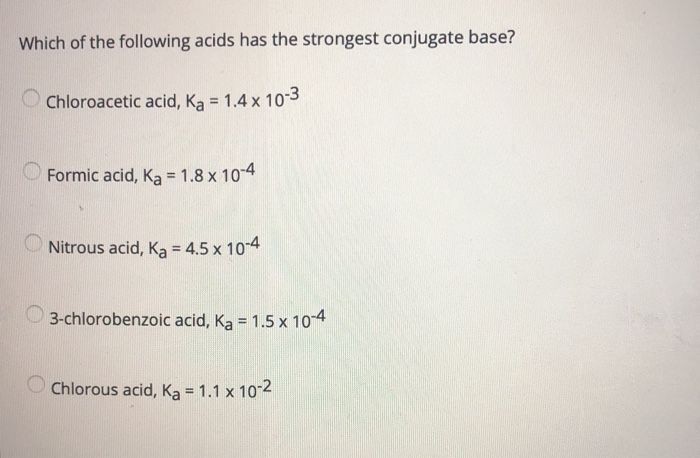 Source: chegg.com
Source: chegg.com
Their reasoning is that because perchloric acid is a strong acid, it will produce a. The pka value for each acid is given in parentheses. Reset help hci of the acids hcl or hf the one with the stronger conjugate base hf hso4 hzs of the acids hzpo4 or hno:
 Source: numerade.com
Source: numerade.com
If hcl is a strong acid, it must be a good proton donor. Remember that the strongest conjugate base has a conjugate acid that is really weak. Arrange the following acids in order from the strongest conjugate base formed to the weakest conjugate base formed.

In the last we can conclude that the strongest acid is hclo$_4$. Oh 3 o + 2 ____ 9. Acetic acid (4.74) ammonium ion (9.3) lodic acid (0.77) nitrous acid (3.16) lactic acid (3.8)
 Source: clutchprep.com
Source: clutchprep.com
The strongest acids ionize 100%. Rather it dissociates partially andestablishes equilibrium with its conjugate base. The acid strength goes up when the base strength goes down.) therefore, the strong acids that are listed have the weakest conjugate bases, removing responses a, d, and e.
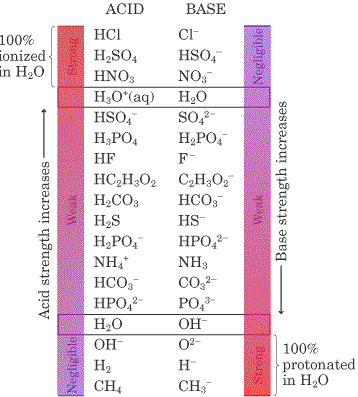 Source: kchemistry.com
Source: kchemistry.com
O acetic acid (4.74) o ammonium ion (9.3) lodic acid (0.77) o nitrous acid (3.16) o lactic acid (3.8) Strong acids have a weak conjugate base. Byjus asked on june 11, 2016 in chemistry.
Also Read :





