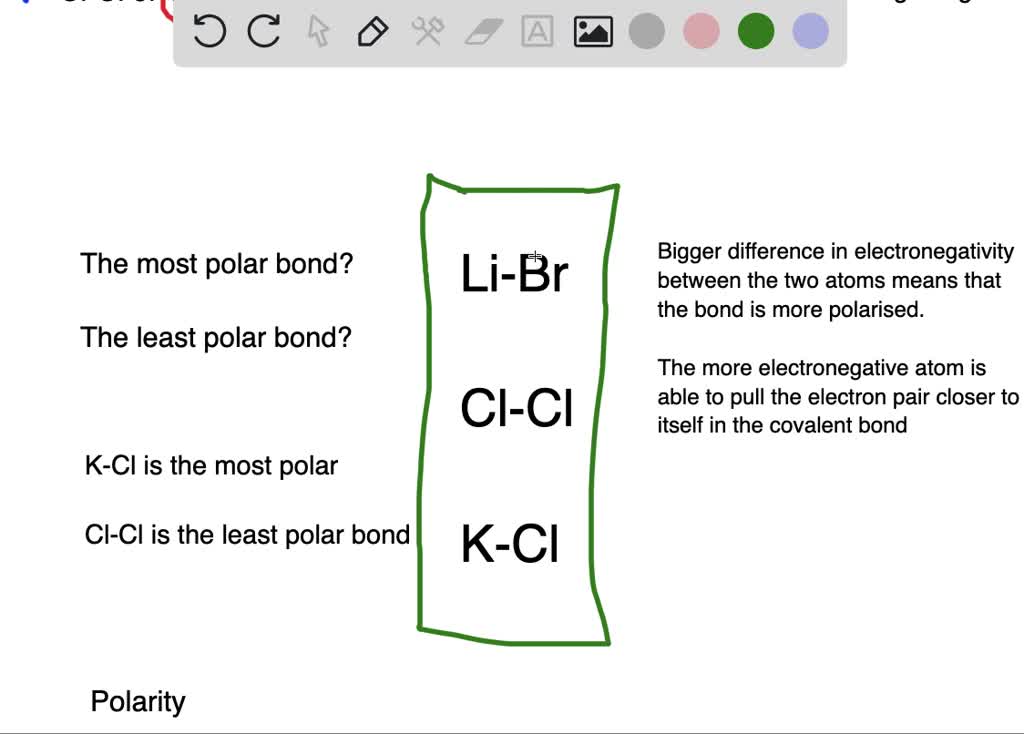
To determine the polarity of a covalent bond using numerical means, find the difference between the electronegativity of the atoms; So the bigger difference in election negativity between the two atoms are bonded means that the bond is more polarized.
Which Is The Most Polar Bond. Bond dissociation energy of f 2 molecule is less than that of c l 2 molecule. These two atoms in brcl would have a nonpolar covalent bond. The polarity of a bond arises when there is a difference in electronegativity between two atoms that are bonded to each other. So when we�re looking at the most polar, we want the biggest difference in electro negativity, which is also known as doubts a try and that drawing.
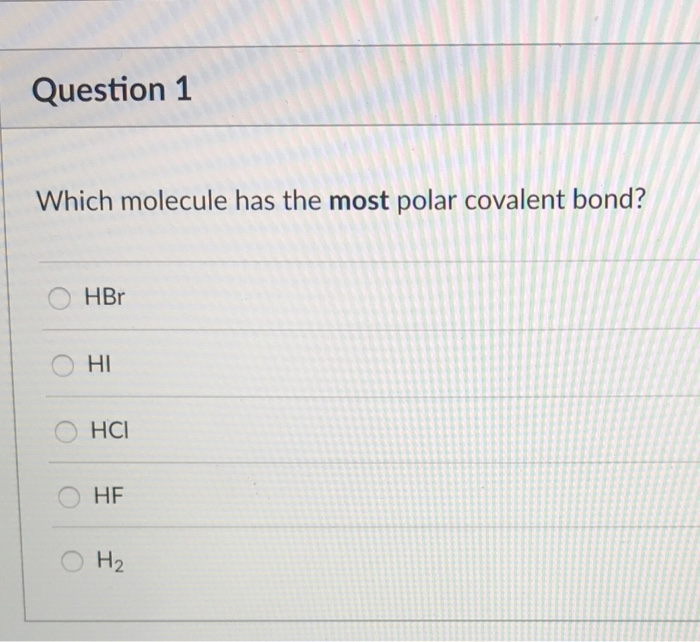 Solved Question 1 Which Molecule Has The Most Polar Covalent | Chegg.com From chegg.com
Solved Question 1 Which Molecule Has The Most Polar Covalent | Chegg.com From chegg.com
Related Post Solved Question 1 Which Molecule Has The Most Polar Covalent | Chegg.com :
Which is the most polar bond in each set? According to the pauling scale,. H2 is nonpolar since the two atoms involved in the bond are identical. Check the electronegativities of the atoms involved in each bond.
So the more electra negative atom is able to pull the electron pair closer towards itself within that cur vaillant bond.
So the bigger difference in election negativity between the two atoms are bonded means that the bond is more polarized. Ccl4 is a nonpolar molecule containing polar covalent bonds. Select the correct order of boiling point. Since electronegativity decreases as you move down groups in the periodic table, a is the correct response. Bond polarity will increase because the electronegativity distinction between the 2 bonding atoms will increase. The higher the electronegativity difference, the more polar a bond is.
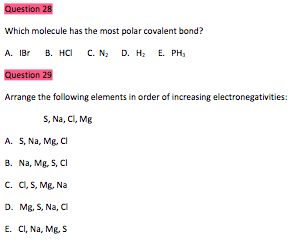 Source: chegg.com
Source: chegg.com
Only given a table of electronegativities, the first level of analysis would be to assume that all the bond lengths were equal and that that the polarity would simply be a function of the difference in electronegativity. Chlorine has a value of 3.0 while bromine has a value of 2.8. The bond which fluorine forms with hydrogen molecule will.
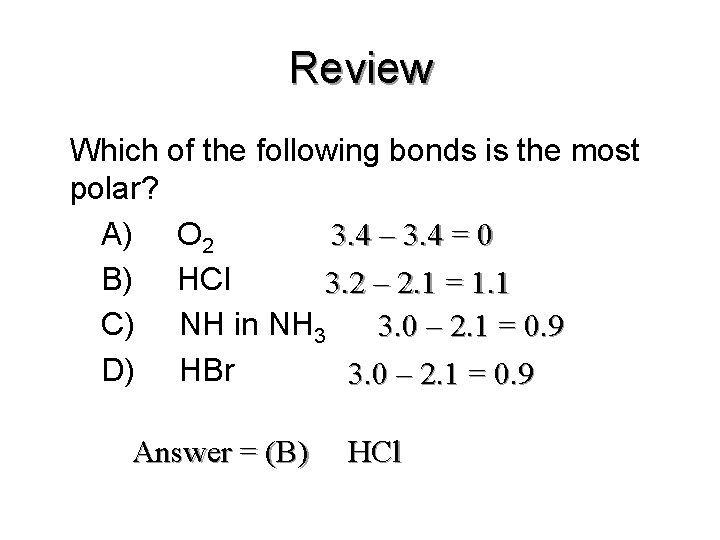 Source: slidetodoc.com
Source: slidetodoc.com
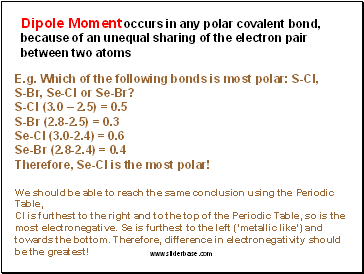
A polar bond is formed between two atoms of different electronegativity. The polarity of a bond is given by the difference in electronegativity between the two atoms that form said bond. The polarity of a bond arises when there is a difference in electronegativity between two atoms that are bonded to each other.
 Source: chegg.com
Source: chegg.com
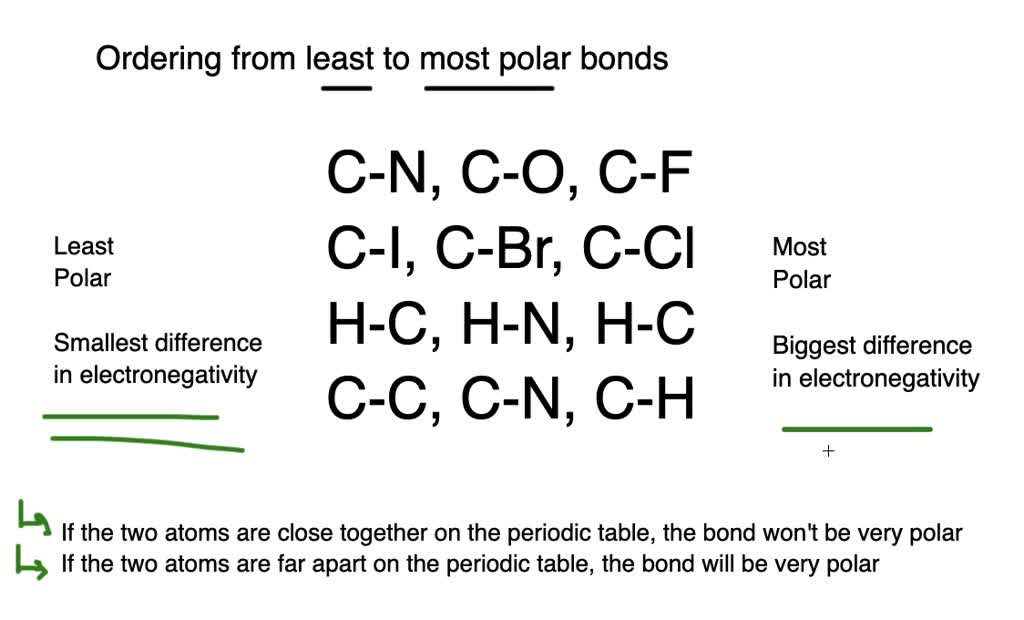
When two elements are next to one another in the periodic table they have similar electronegativities. Bond dissociation energy of f 2 molecule is less than that of c l 2 molecule. In summary, it’s essentially impossible to give you an answer as to the most polar bond, as bonding can be considered a spectrum between 100% covalent and 100% ionic.
 Source: numerade.com
Source: numerade.com
How do you tell which covalent bond is most polar? Technically ionic bonds are completely polar bonds, so the terminology can be confusing. How do you know which bond is more polar?
 Source: chegg.com
Source: chegg.com
The polarity of a bond is given by the difference in electronegativity between the two atoms that form said bond. In summary, it’s essentially impossible to give you an answer as to the most polar bond, as bonding can be considered a spectrum between 100% covalent and 100% ionic. Recall that for a covalent bond to be:
 Source: chegg.com
Source: chegg.com
Chlorine has a value of 3.0 while bromine has a value of 2.8. The bond which fluorine forms with hydrogen molecule will. H2 is nonpolar since the two atoms involved in the bond are identical.
 Source: sliderbase.com
Source: sliderbase.com
Bond dissociation energy of f 2 molecule is less than that of c l 2 molecule. Fluorine is the most electronegative element in the periodic table therefore; Technically ionic bonds are completely polar bonds, so the terminology can be confusing.
 Source: chemistrytalk.org
Source: chemistrytalk.org
Bond polarity will increase because the electronegativity distinction between the 2 bonding atoms will increase. H2 is nonpolar since the two atoms involved in the bond are identical. In simple words, a bond polarity is a scientific tool that gives us an idea about the nature of the bonds and the type of bonding they will undergo to form compounds.
 Source: numerade.com
Source: numerade.com
So the bigger difference in election negativity between the two atoms are bonded means that the bond is more polarized. Bond polarity will increase because the electronegativity distinction between the 2 bonding atoms will increase. The nf bond is more polar than the nh bond:
 Source: numerade.com
Source: numerade.com
Atoms with similar en polar covalent bonds: Check the electronegativities of the atoms involved in each bond. Electronegativity is a measure of how.
 Source: itprospt.com
Source: itprospt.com
This value means that fluorine in the molecul hf, also known as hydrofluoric acid, is a. The greatest difference in electronegativity will correspond to the most polar bond. People also ask, which bond is most polar?
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
But nf3 has a smaller dipole moment than nh3. But nf3 has a smaller dipole moment than nh3. When two elements are next to one another in the periodic table they have similar electronegativities.
 Source: youtube.com
Source: youtube.com
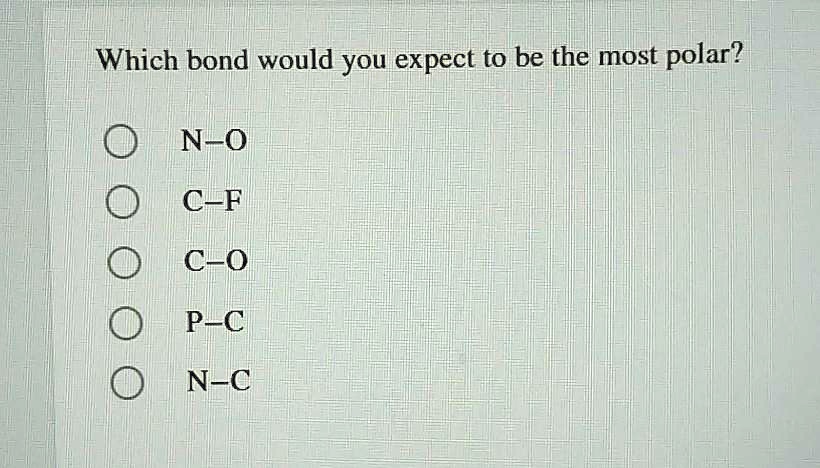
Bond polarity and inductive effectbond polarity and inductive effect nonpolar covalent bonds: Based on electronegativity values, c―o bond is the most polar one in the above options. Check the electronegativities of the atoms involved in each bond.
 Source: youtube.com
Source: youtube.com
Those atoms having high difference of electronegativity values are more polar as compared to those having less difference of electronegativity values. The bond which fluorine forms with hydrogen molecule will. Ccl4 is a nonpolar molecule containing polar covalent bonds.
 Source: clutchprep.com
Source: clutchprep.com
To determine the polarity of a covalent bond using numerical means, find the difference between the electronegativity of the atoms; The polarity of a bond is given by the difference in electronegativity between the two atoms that form said bond. According to the pauling scale,.
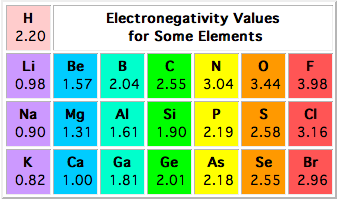 Source: socratic.org
Source: socratic.org
How do you know which bond is more polar? Fluorine is the most electronegative element in the periodic table therefore; Which bond is more polar h cl or ho?
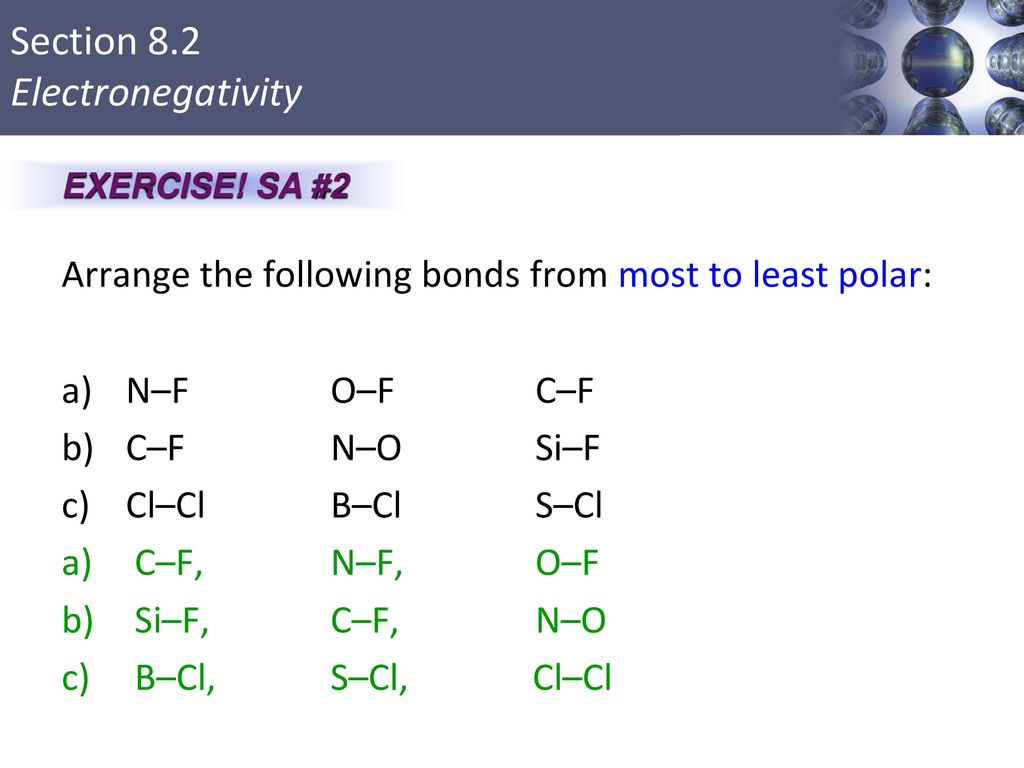 Source: slideplayer.com
Source: slideplayer.com
In the case of hf, hydrogen has an en of 2.1 and fluorine an en of 4.0 (highest en of any element). Is hf the most polar bond? In summary, it’s essentially impossible to give you an answer as to the most polar bond, as bonding can be considered a spectrum between 100% covalent and 100% ionic.
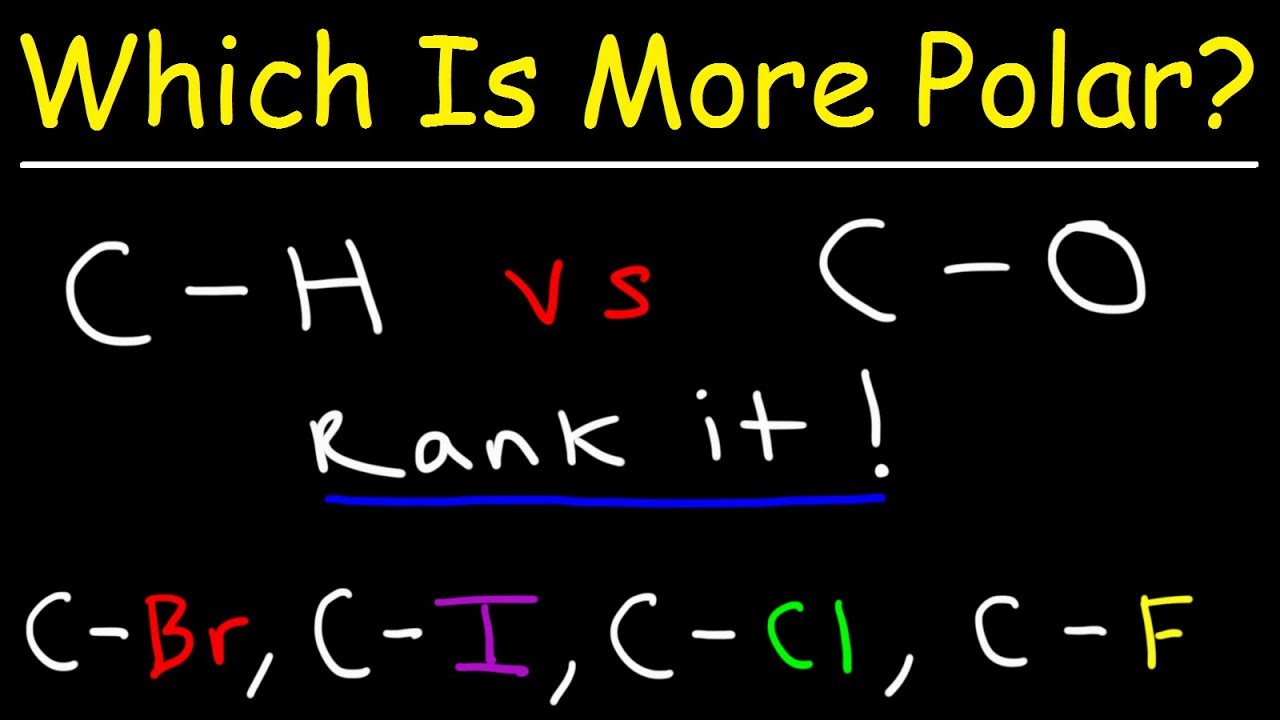 Source: youtube.com
Source: youtube.com
The molecule with the polar bond that has the greatest difference in electronegativity is the most polar. In summary, it’s essentially impossible to give you an answer as to the most polar bond, as bonding can be considered a spectrum between 100% covalent and 100% ionic. H2 is nonpolar since the two atoms involved in the bond are identical.
 Source: clutchprep.com
Source: clutchprep.com
How do you know which bond is more polar? Bond polarity will increase because the electronegativity distinction between the 2 bonding atoms will increase. The higher the electronegativity difference, the more polar a bond is.
 Source: slidetodoc.com
Source: slidetodoc.com
H2 is nonpolar since the two atoms involved in the bond are identical. Polarity of substance is depend on difference of electronegativity values between bonded atoms. H2 is nonpolar since the two atoms involved in the bond are identical.
Also Read :