Check answer and solution for above que The hydroxyl proton is the most acidic.
Which Is The Most Acidic Proton In The Following Compound. Which is the most acidic proton in the following compound? All groups are equally acidic is the most acidic. Which is the most acidic proton in the molecule shown below? Which of the following compounds would undergo racemization in the presence of a base?
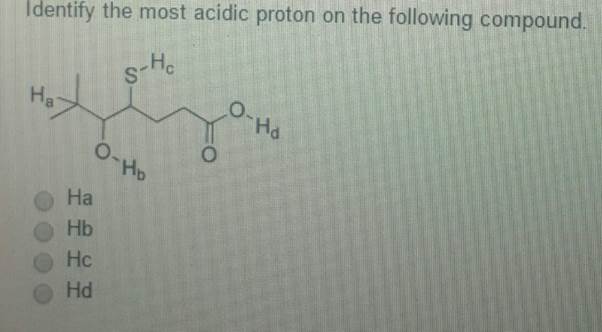
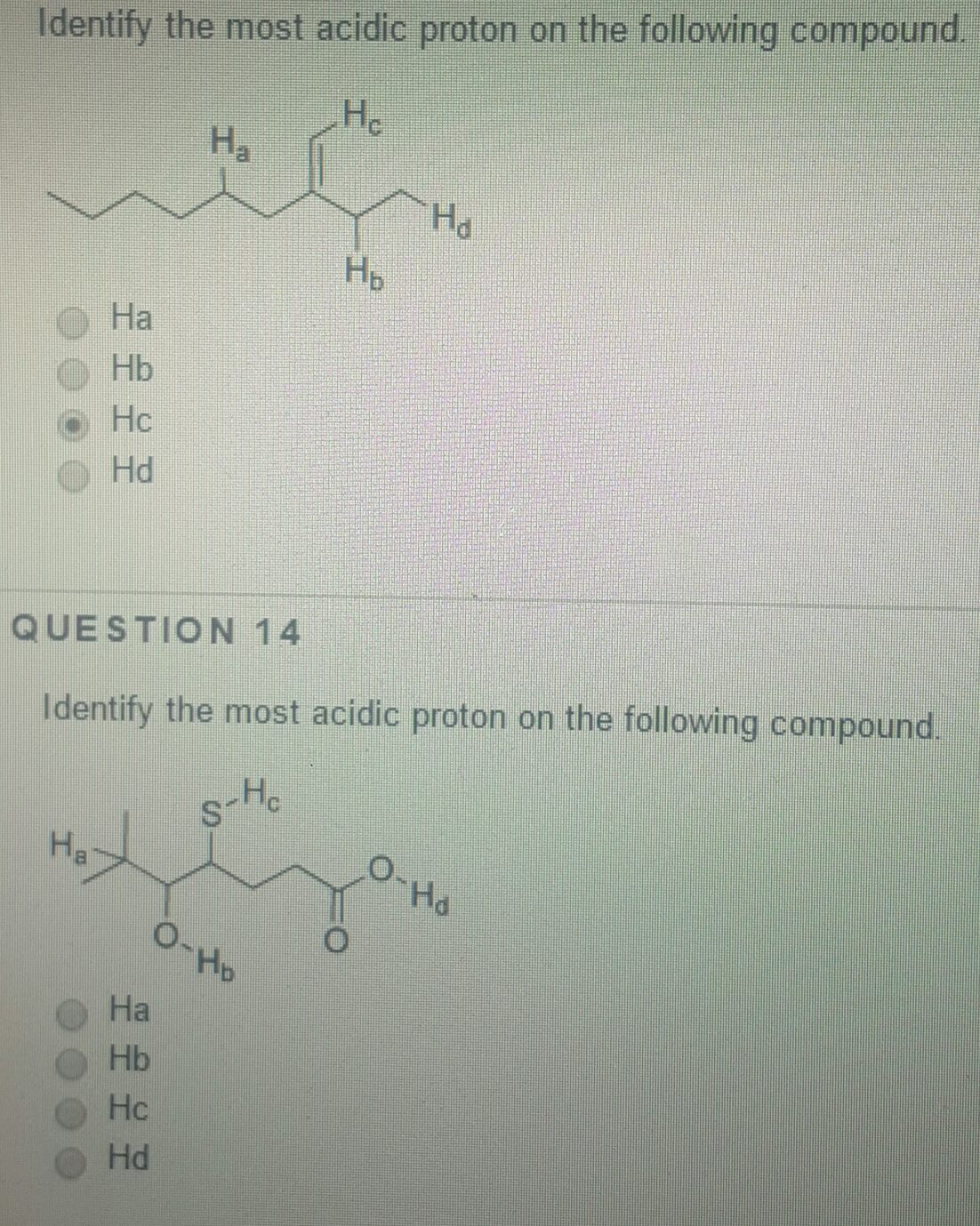
 Oneclass: Identify The Most Acidic Proton On The Following Compound. A) Ha B) Hb C) Hc D) Hd E) Hb An… From oneclass.com
Oneclass: Identify The Most Acidic Proton On The Following Compound. A) Ha B) Hb C) Hc D) Hd E) Hb An… From oneclass.com
Related Post Oneclass: Identify The Most Acidic Proton On The Following Compound. A) Ha B) Hb C) Hc D) Hd E) Hb An… :
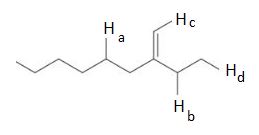
A) ha b) hb c) hc d) hd e) hb and hd are equally acidic Which is the most acidic proton in the following compound? Diphenylmethane is significantly more acidic than benzene, and triphenylmethane is more acidic than either. Identify the most acidic proton on the following compound.
Ah, and so this will be the least acidic proton in this molecule.
The most acidic functional group usually is holding the most acidic h in the entire molecule. Um, but still less acidic than be so a is gonna be the intermediate acidity here. Diphenylmethane is significantly more acidic than benzene, and triphenylmethane is more acidic than either. Ha hb hc hd identify the most acidic proton on the following compound. All groups are equally acidic is the most acidic. Identify the most acidic proton in each compound, and suggest a reason for the trend in.
 Source: oneclass.com
Source: oneclass.com
C h 3 c o o h → s o c l 2 a → a n h y. So we would predict that this proton will have a pka much higher than the normal alkenes (pka >> 45). The most acidic proton is one that is between atoms f and cl.
 Source: chegg.com
Source: chegg.com
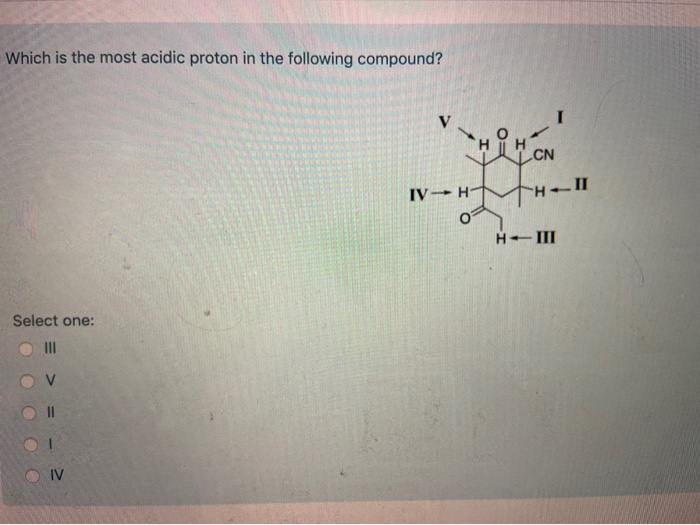
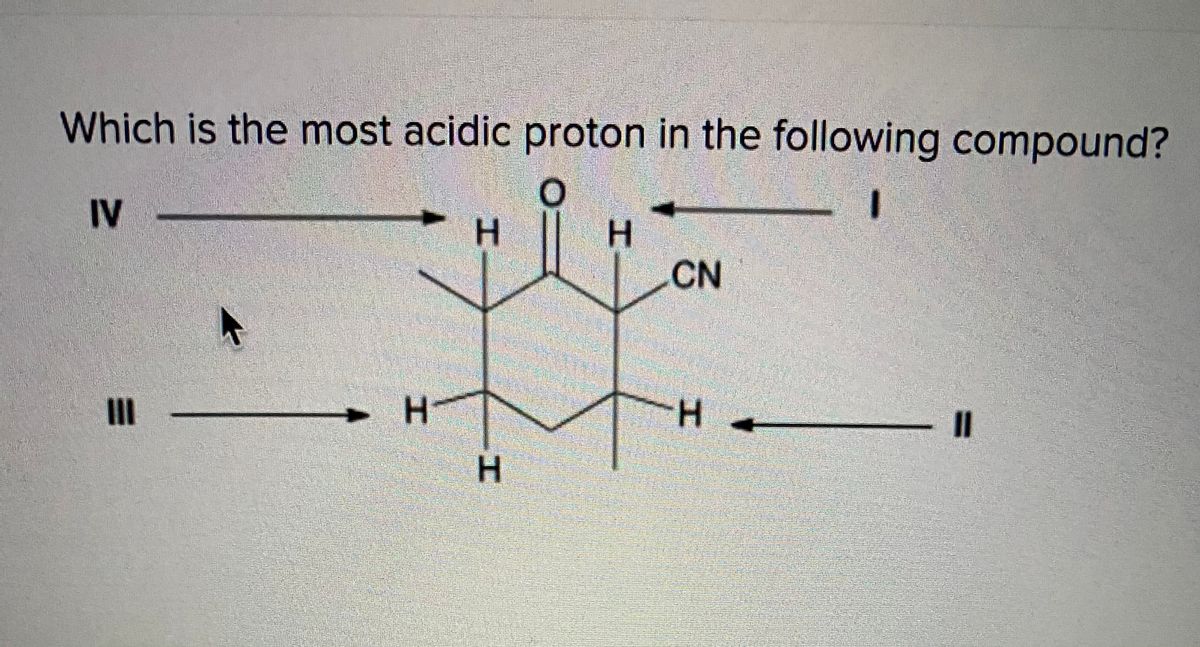
Which of the following compounds would undergo racemization in the presence of a base? Among the following, the compound having the most acidic proton is: X is an alkyl proton adjacent to a carbonyl.
 Source: chegg.com
Source: chegg.com
Therefore option d is the most acidic where f is adjacent to hydroxylic proton. Which is the most acidic proton in the molecule shown below? Identify the most acidic proton on the following compound.
 Source: numerade.com
Source: numerade.com
Proton (b) is the most acidic Which of the indicated protons is most acidic? Um, but still less acidic than be so a is gonna be the intermediate acidity here.
 Source: transtutors.com
Source: transtutors.com
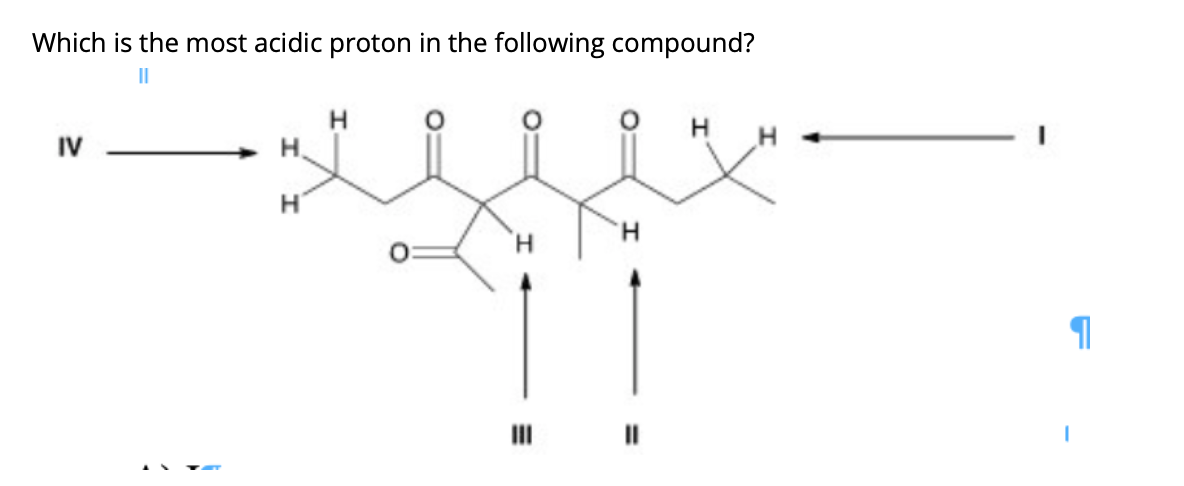
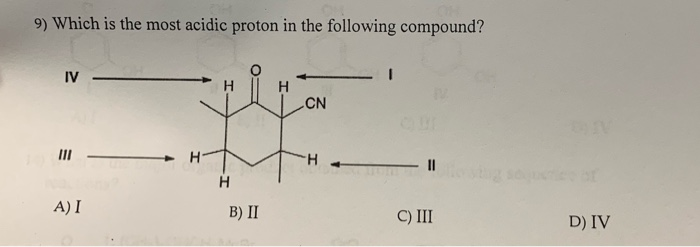
A) i b) ii c) iii d) iv 34. Thus extricating hb is more feasible thus making it more acidic. X is an alkyl proton adjacent to a carbonyl.
 Source: bartleby.com
Source: bartleby.com
Determine if naoh is a suitable reagent to deprotonate the following compound. In a set of reactions, acetic acid yielded product d. The methyl proton is the most acidic.
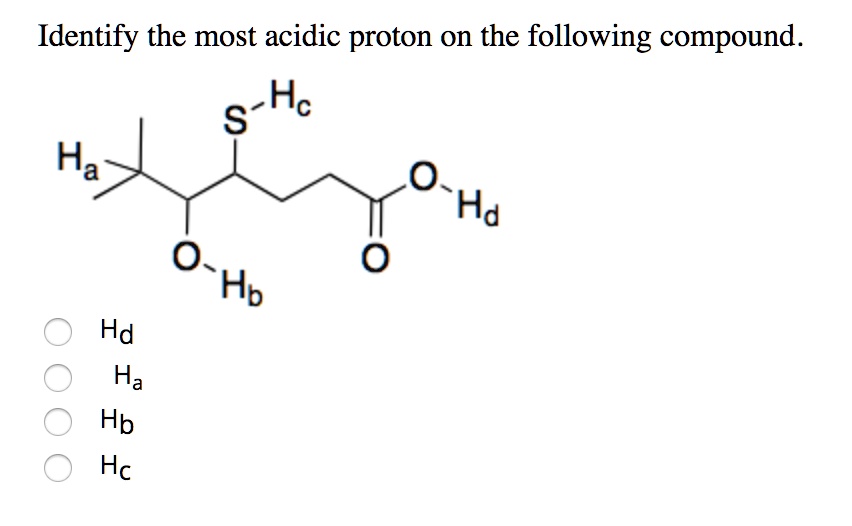 Source: itprospt.com
Source: itprospt.com
Um proton, eh, uh, is on an alfa carbon. All groups are equally acidic is the most acidic. We need to comfort the protons of the given compound and decide which one is more acidic.
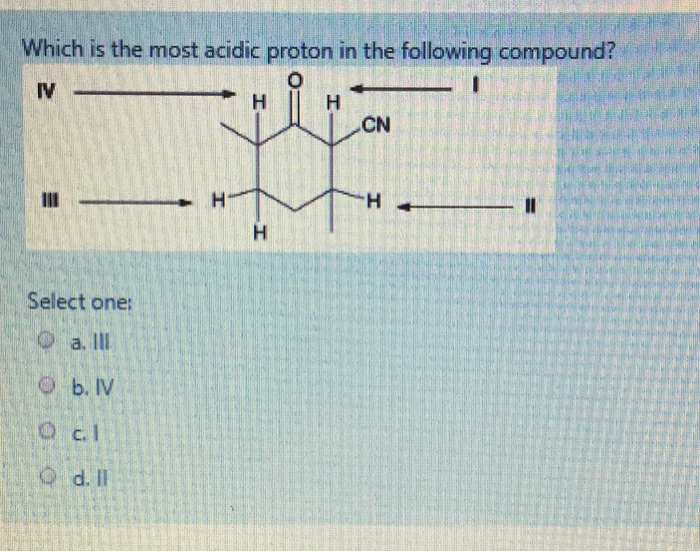 Source: chegg.com
Source: chegg.com
Therefore, p should be the most acidic. Only select the proton, not the bond. Among the following, the compound having the most acidic proton is:
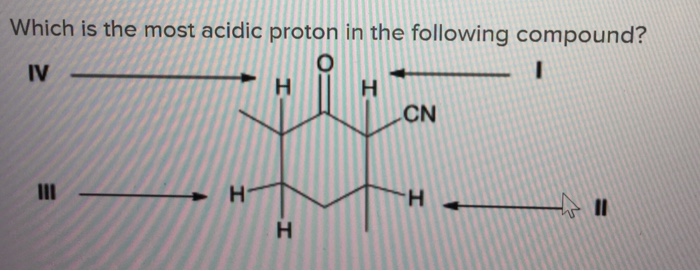
For that, we need to get the country get base by pulling off one proton because the more acidic proton is that one which leaves to give the most stable, contradict base. Which of the following compounds would undergo racemization in the presence of a base? Which is the most acidic proton in the molecule shown below?
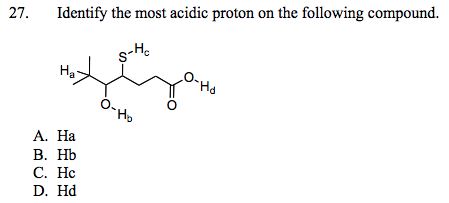 Source: chegg.com
Source: chegg.com
Um, but still less acidic than be so a is gonna be the intermediate acidity here. The most acidic functional group usually is holding the most acidic h in the entire molecule. C h 3 c o o h → s o c l 2 a → a n h y.
 Source: oneclass.com
Source: oneclass.com
Identify the most acidic site in thesecompounds: Which of the following compounds is most acidic? Aldehydes ketones and carboxylic acids.
 Source: chegg.com
Source: chegg.com
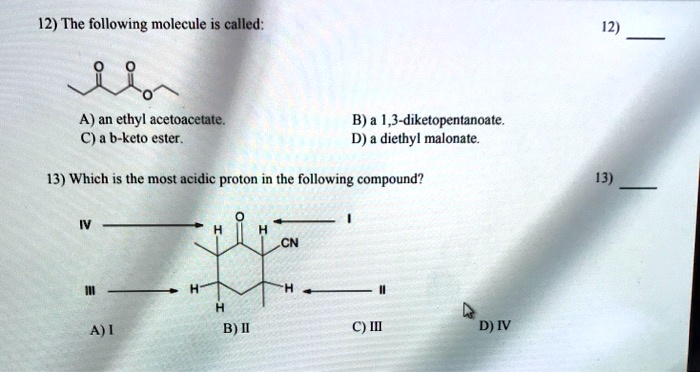
Identify the most acidic proton on the following compound. Thus extricating hb is more feasible thus making it more acidic. The methyl proton is the most acidic.
 Source: solvedlib.com
Source: solvedlib.com
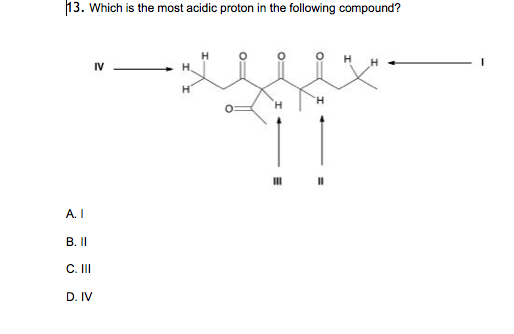
Among the following, the compound having the most acidic proton is: X is an alkyl proton adjacent to a carbonyl. The proton that is between atoms f and br follows.
 Source: chegg.com
Source: chegg.com
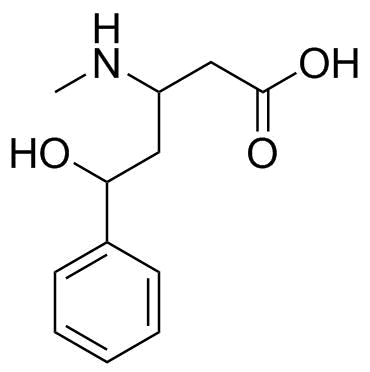
Which of the following compounds is most acidic? Which proton in each of the following drugs is most acidic? Identify the most acidic proton on the following compound.
 Source: shimizu-uofsc.net
Source: shimizu-uofsc.net
P is an amide proton. Therefore option d is the most acidic where f is adjacent to hydroxylic proton. C h 3 c o c h 2 c o c h 3.
 Source: study.com
Source: study.com
A l c l 3 b e n z e n e b → h c n c → h o h d. Which of the following compounds is most acidic? A) ha b) hb c) hc d) hd e) hb and hd are equally acidic
 Source: sites.science.oregonstate.edu
Source: sites.science.oregonstate.edu
Which is the most acidic proton in the molecule shown below? There are two of them. Its conjugate base is the weakest base here, and is thus the most.
 Source: study.com
Source: study.com
Which of the following compounds would undergo racemization in the presence of a base? The amino proton is the most acidic. Proton (b) is the most acidic
 Source: chegg.com
Source: chegg.com
Identify the most acidic proton in each of the following compounds: The amino proton is the most acidic. Thus extricating hb is more feasible thus making it more acidic.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
A l c l 3 b e n z e n e b → h c n c → h o h d. P is an amide proton. All groups are equally acidic is the most acidic.
Also Read :





