But carbocation #5 is vinylic carbocation (positively charged carbon is sp 2 hybridized, i.e. Carbocation (c) is antiaromatic and hence is least stable.
Which Is The Least Stable Carbocation. Is a carbocation sp2 or sp3? So, the tertiary carbocation is the most stable, and the least is the methyl carbocation. Carbocation (c) is antiaromatic and hence is least stable. C < a < b what is the expected major product(s) of hcl addition to the alkene below?
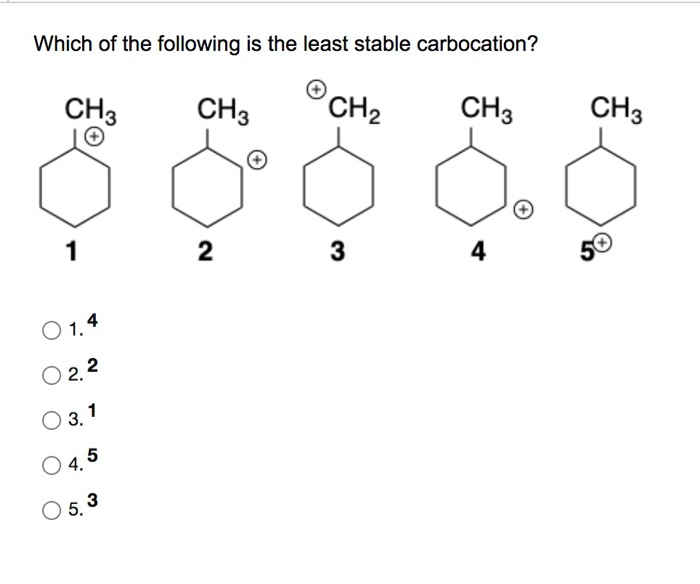 Solved Which Of The Following Is The Least Stable | Chegg.com From chegg.com
Solved Which Of The Following Is The Least Stable | Chegg.com From chegg.com
Related Post Solved Which Of The Following Is The Least Stable | Chegg.com :
Carbocations are hypovalent species which have only three shared pairs of electrons around the carbon, instead of the usual four. Then, which is the least stable carbocation? C h 3c h ⋅ 2 c h 3 c h 2 ⋅. Which carbocation intermediate is least stable?
So (4) is least stable configuration.
Is a carbocation sp2 or sp3? Structure (a) is the least stable resonating structure because in this structure the negative charge is present on the carbon atom attached −nh2 group which is electron donating in nature which will destabilize the molecule. Then, which is the least stable carbocation? Which of the following is the least stable carbocation? The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. A vinylic carbocation carries the positive charge on an sp carbon, which is more electronegative than an sp2 carbon of an alkyl carbocation.
 Source: oneclass.com
Source: oneclass.com
(c h 3)3c ⋅ ( c h 3) 3 c ⋅. Tripheny carbocation only stabilised by resonance so it�s least stable than 3°. In any event, the methyl carbocation will be the least stable carbocation of all.
![Solved] Rank The Following Carbocations In Order Of Most Stable To Least Stable. | Course Hero](https://www.coursehero.com/qa/attachment/13732853/ “Solved] Rank The Following Carbocations In Order Of Most Stable To Least Stable. | Course Hero”) Source: coursehero.com
Ncert dc pandey sunil batra hc verma pradeep errorless. Further out of (b) and (d), (d) has less angular strain and as such is. (c h 3)2c h ⋅ ( c h 3) 2 c h ⋅.
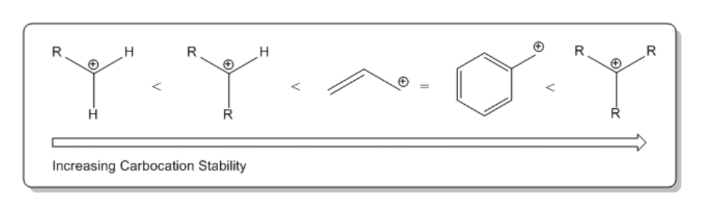 Source: clutchprep.com
Source: clutchprep.com
What is the least stable resonating structure? (c h 3)3c ⋅ ( c h 3) 3 c ⋅. Therefore, option (b) is correct.
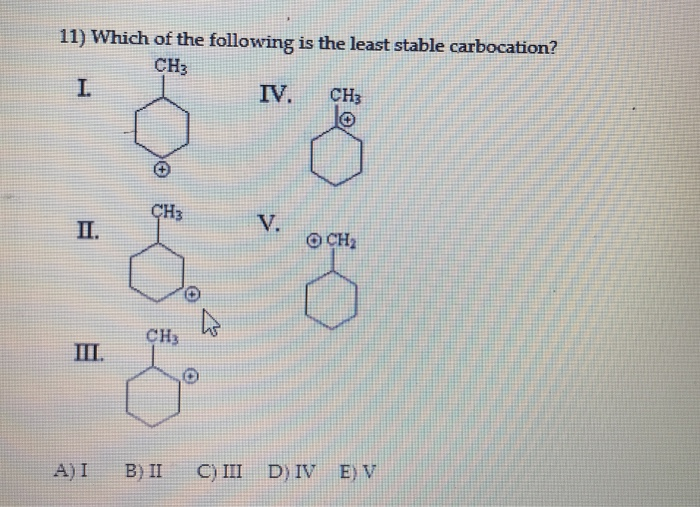 Source: chegg.com
Source: chegg.com
C < a < b what is the expected major product(s) of hcl addition to the alkene below? So (4) is least stable configuration. But the most stable is tricyclo propane carbocation because it�s have bendin p.
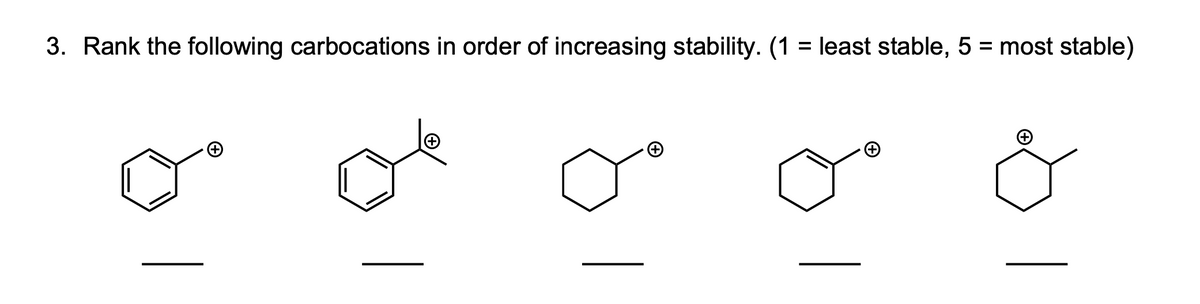 Source: bartleby.com
Source: bartleby.com
Least stable carbocation among the following is. Answered oct 24, 2019 by hitheshkumar (85.2k points) best answer. Which carbocation is most reactive?
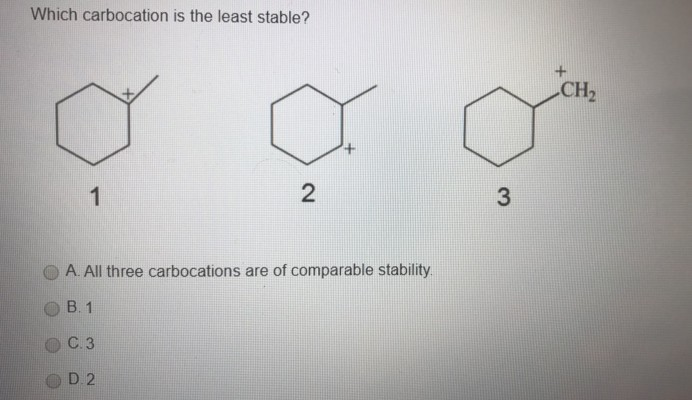 Source: clutchprep.com
Source: clutchprep.com
So, the tertiary carbocation is the most stable, and the least is the methyl carbocation. ‘all carbocations are strong alkylating agents with a high affinity for groups which donate or share their electrons, such as halide ions, hydroxyl anions and amines.’ ‘the resulting esters generate carbocations that react with dna.’ ‘the methyl carbocation, ch. The more stable the carbocation, the lower the activation energy for reaching that intermediate will be.
 Source: oneclass.com
Source: oneclass.com
With three bond pairs and zero lone pairs it has trigonal planar geometry by vsepr theory, and trigonal planar is characteristic of sp2 hybridisation. Therefore a primary vinylic carbocation is less stable than a primary alkyl carbocation. Answered oct 24, 2019 by hitheshkumar (85.2k points) best answer.
![Solved] 3) Which Of The Following Is The Most Stable Carbocation? C) D) B) A) 4) Which Of The Following Is The Least Stable Carbocation? D) C) B) A)… | Course Hero](https://www.coursehero.com/qa/attachment/11247791/ “Solved] 3) Which Of The Following Is The Most Stable Carbocation? C) D) B) A) 4) Which Of The Following Is The Least Stable Carbocation? D) C) B) A)… | Course Hero”) Source: coursehero.com
Ncert dc pandey sunil batra hc verma pradeep errorless. Which carbocation below is the least stable? (c h 3)3c ⋅ ( c h 3) 3 c ⋅.
 Source: study.com
Source: study.com
1 2 3 4 12) (6 pts.) rank the following alkenes from most to least stable. Then, which is the least stable carbocation? C h 3c h ⋅ 2 c h 3 c h 2 ⋅.
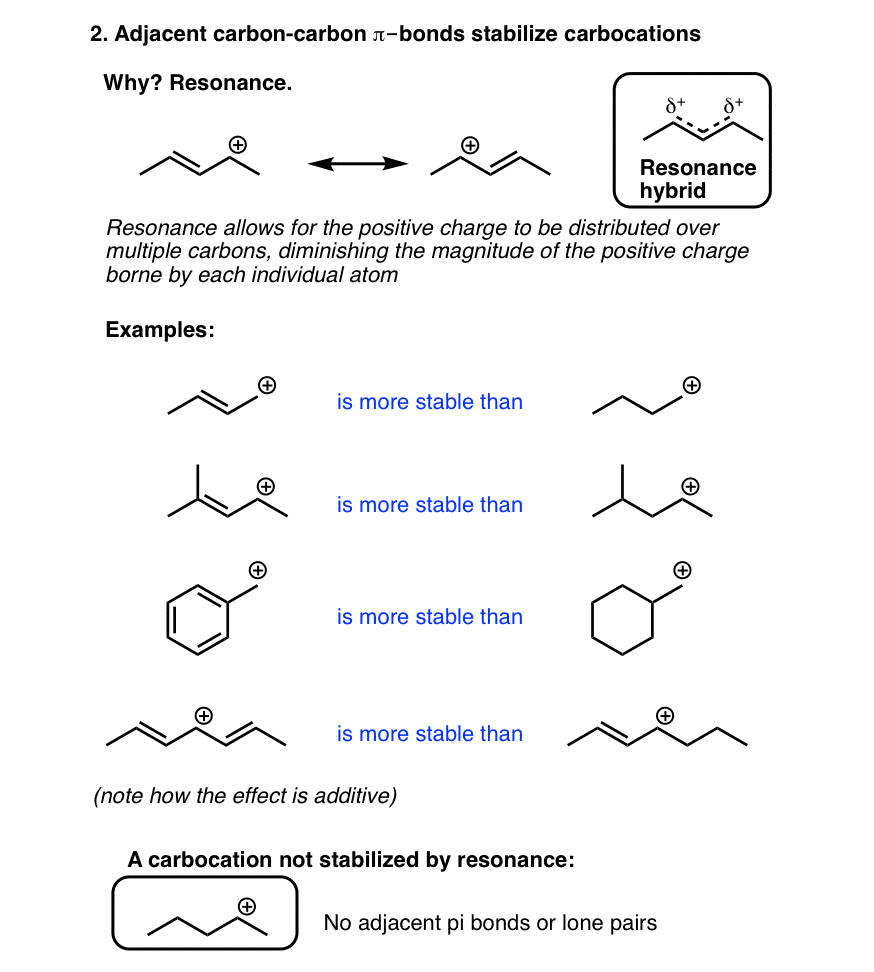 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
(c h 3)2c h ⋅ ( c h 3) 2 c h ⋅. Further out of (b) and (d), (d) has less angular strain and as such is. So, it will be the least stable species.

Carbocation (c) is antiaromatic and hence is least stable. But carbocation #5 is vinylic carbocation (positively charged carbon is sp 2 hybridized, i.e. Tripheny carbocation only stabilised by resonance so it�s least stable than 3°.
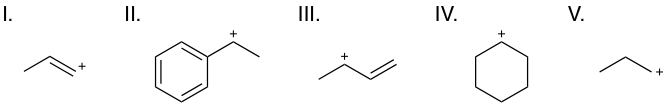 Source: varsitytutors.com
Source: varsitytutors.com
What is the least stable resonating structure? With three bond pairs and zero lone pairs it has trigonal planar geometry by vsepr theory, and trigonal planar is characteristic of sp2 hybridisation. Therefore a primary vinylic carbocation is less stable than a primary alkyl carbocation.

Therefore, option (b) is correct. Least stable carbocation among the following is. As we all know, the carbocation will be the most stable if the carbocation is most substituted.
 Source: chegg.com
Source: chegg.com
So (4) is least stable configuration. With three bond pairs and zero lone pairs it has trigonal planar geometry by vsepr theory, and trigonal planar is characteristic of sp2 hybridisation. Which carbocation intermediate is least stable?
 Source: toppr.com
Source: toppr.com
So, it will be the least stable species. What is the least stable resonating structure? Therefore a primary vinylic carbocation is less stable than a primary alkyl carbocation.
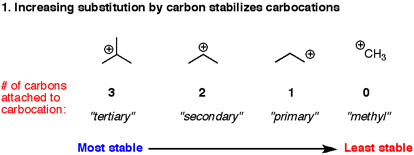 Source: socratic.org
Source: socratic.org
But the most stable is tricyclo propane carbocation because it�s have bendin p. Therefore (d) and (b) are more stable than (a). With three bond pairs and zero lone pairs it has trigonal planar geometry by vsepr theory, and trigonal planar is characteristic of sp2 hybridisation.
 Source: itprospt.com
Source: itprospt.com
But the most stable is tricyclo propane carbocation because it�s have bendin p. C h 3c h ⋅ 2 c h 3 c h 2 ⋅. Carbocation (a), (b) and _ (d) are all secondary but (d) and (b) are aromatic.
 Source: chemistryscore.com
Source: chemistryscore.com
Carbocations are hypovalent species which have only three shared pairs of electrons around the carbon, instead of the usual four. What is the least stable resonating structure? Is a carbocation sp2 or sp3?
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
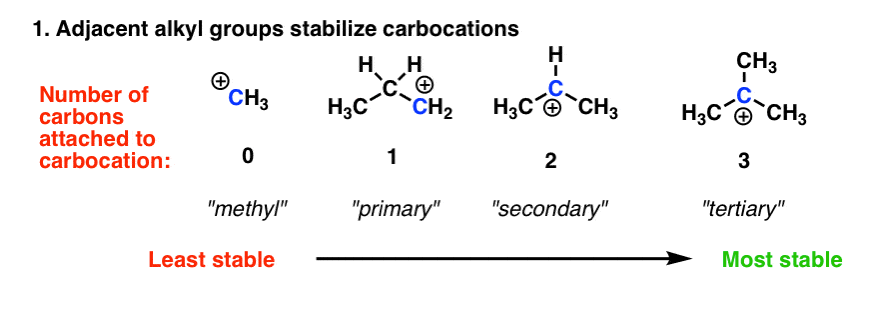
What is the least stable resonating structure? The least stable carbocation is the carbocation (a) as an electron withdrawing group destabilizes carbocation by intensifying the positive charge. The carbocation #1 is a saturated carbocation which is stabilized by hyperconjugation.
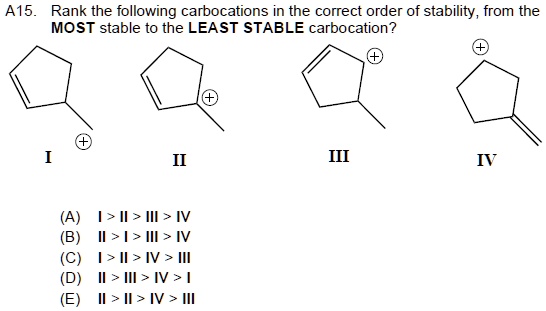 Source: numerade.com
Source: numerade.com
‘all carbocations are strong alkylating agents with a high affinity for groups which donate or share their electrons, such as halide ions, hydroxyl anions and amines.’ ‘the resulting esters generate carbocations that react with dna.’ ‘the methyl carbocation, ch. So, it will be the least stable species. Answered oct 24, 2019 by hitheshkumar (85.2k points) best answer.
Also Read :





