Assume that each of the ions in the nacl solution has the same effect on the freezing point of water as a nonelectrolyte molecule, and determine the freezing temperature the solution (which is approximately. Principle dissolved ions in seawater 19 3.
Which Ions Comprise About 85 Of The Solutes In Seawater. About 80% of the total particulate carbon flux through the thermocline is in the form of organic matter. What are the top 5 elements in seawater? Possible brine origins 51 6. D)two hydrogen atoms of the same molecule.
 Pdf) Salt-Regulated Accumulation Of The Compatible Solutes Sucrose And Glucosylglycerol In Cyanobacteria And Its Biotechnological Potential From researchgate.net
Pdf) Salt-Regulated Accumulation Of The Compatible Solutes Sucrose And Glucosylglycerol In Cyanobacteria And Its Biotechnological Potential From researchgate.net
Related Post Pdf) Salt-Regulated Accumulation Of The Compatible Solutes Sucrose And Glucosylglycerol In Cyanobacteria And Its Biotechnological Potential :
They come from the atmosphere and dissolves in seawater at the sea surface. The ions can pass out and the large proteins remain. Bicarbonate ions constitute 48% of river water solutes but only 0.14% for seawater. By weight these ions make up about 99 percent of all sea salts.
A)ions in solution between the molecules.
They come from the atmosphere and dissolves in seawater at the sea surface. Sodium and chloride have very long residence times, while calcium (vital for carbonate formation) tends to. The table below gives the concentration of some ions in the sample. Ionic contents resulting from dissolution of modified marine solutes with seawater 58 8. C)hydrogen and oxygen atoms of adjacent molecules. What are the top 5 elements in seawater?
 Source:
Source:
Base your answers to questions 76 through 79 on the information below and on your knowledge of chemistry. Oxygen, carbon dioxide, and nitrogen are typically dissolved in the ocean. *although ocean salinities vary from one part of the ocean to another, forchhammer discovered that salt ions are always present in the same proportions.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
Om is the principle chemical form in which solar energy is made, stored, and used on earth (source of fossil fuels). Two of the most prevalent ions in seawater are chloride and sodium. *although ocean salinities vary from one part of the ocean to another, forchhammer discovered that salt ions are always present in the same proportions.
 Source: researchgate.net
Source: researchgate.net
Magnesium and sulfate make up another 10 percent of the total. About 80% of the total particulate carbon flux through the thermocline is in the form of organic matter. We study organic matter in seawater because:
 Source: slideplayer.com
Source: slideplayer.com
Two of the most prevalent ions in seawater are chloride and sodium. Om is the principle chemical form in which solar energy is made, stored, and used on earth (source of fossil fuels). Sodium and chloride have very long residence times, while calcium (vital for carbonate formation) tends to.
 Source: slideshare.net
Source: slideshare.net
Sodium and chloride have very long residence times, while calcium (vital for carbonate formation) tends to. They come from the atmosphere and dissolves in seawater at the sea surface. Magnesium and sulfate make up another 10 percent of the total.
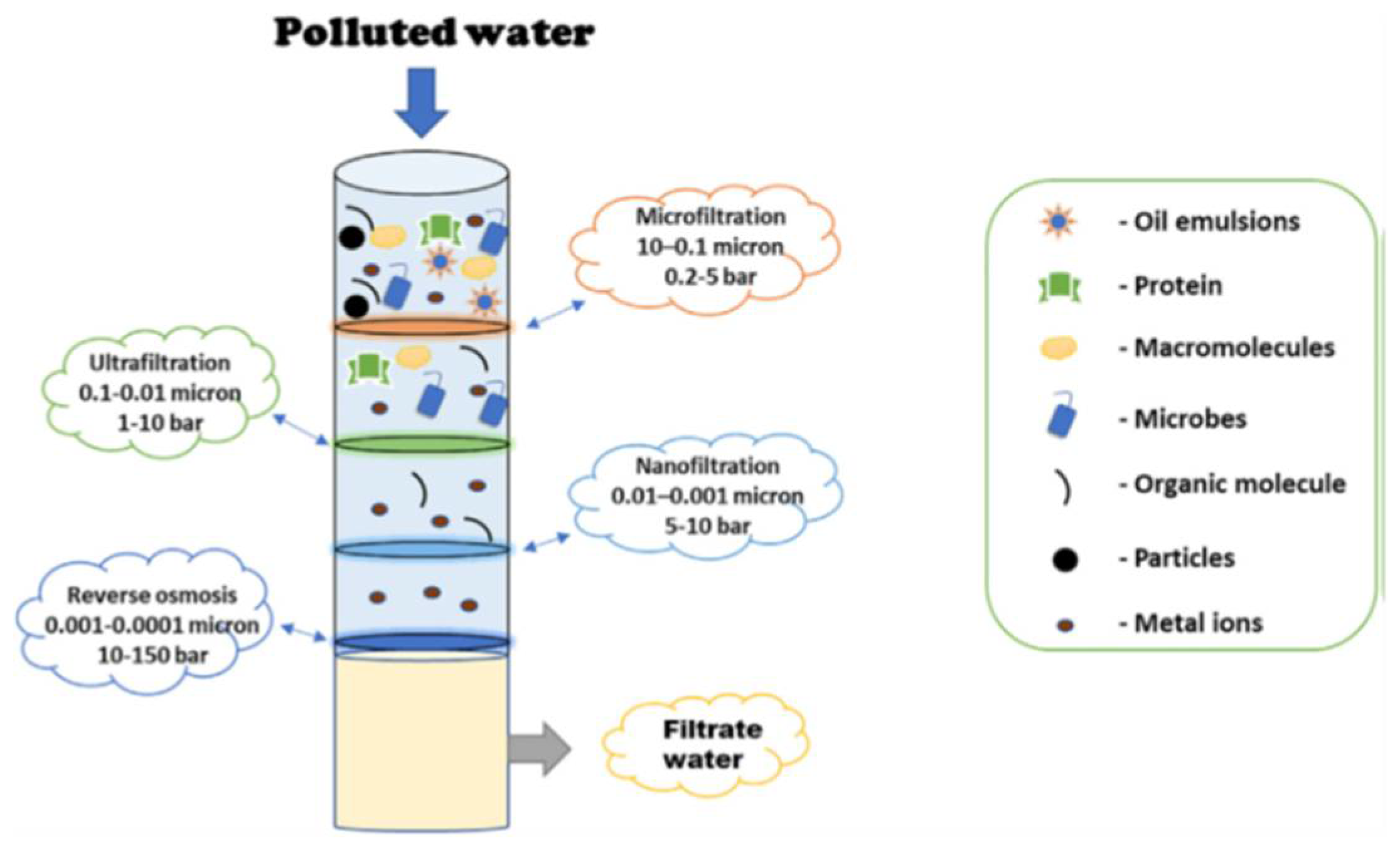 Source: mdpi.com
Source: mdpi.com
Om is the principle chemical form in which solar energy is made, stored, and used on earth (source of fossil fuels). The big 6 of major components of seawater 8. The concentration of salt in seawater (salinity) varies with temperature, evaporation, and precipitation.
 Source: quizlet.com
Source: quizlet.com
Differences like these are due to the varying residence times of seawater solutes; What are the top 5 elements in seawater? What are the 2 sources of the elements in seawater?
 Source: researchgate.net
Source: researchgate.net
Om is the principle chemical form in which solar energy is made, stored, and used on earth (source of fossil fuels). Possible brine origins 51 6. Wang and chen (1983) found liquid bitterns to be effective and economical coagulants for color removal in pulp and.
 Source: fliphtml5.com
Source: fliphtml5.com
The six most abundant ions of seawater are chloride (cl −), sodium (na +), sulfate (so 2 4 −), magnesium (mg 2+), calcium (ca 2+), and potassium (k +). Other ions are found in very small concentrations. Sodium and chloride comprise over 85% of the seawater solutes explain forchhammer�s principle or the principle of constant proportions the percentage of various salts in seawater in the same in samples from many places, regardless of how salty the water is
 Source: researchgate.net
Source: researchgate.net
*although ocean salinities vary from one part of the ocean to another, forchhammer discovered that salt ions are always present in the same proportions. Together, they make up around 85 percent of all dissolved ions in the ocean. Om is the principle chemical form in which solar energy is made, stored, and used on earth (source of fossil fuels).
 Source: slideplayer.com
Source: slideplayer.com
A solvent is a major component in a solution. Two of the most prevalent ions in seawater are chloride and sodium. Salts derived from seawater evaporation 21 4.
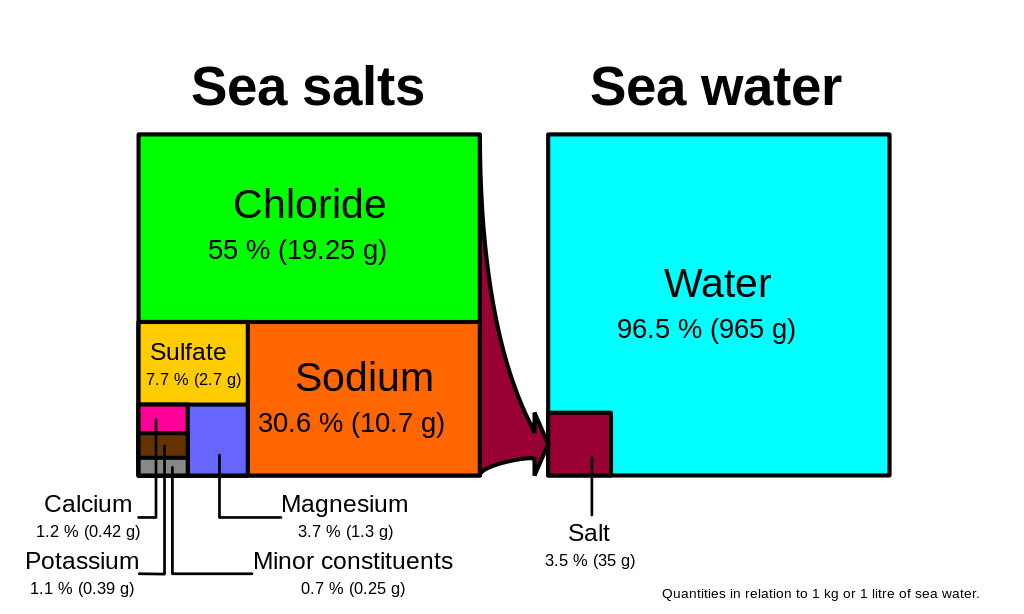 Source: rwu.pressbooks.pub
Source: rwu.pressbooks.pub
What are the top 5 elements in seawater? *although ocean salinities vary from one part of the ocean to another, forchhammer discovered that salt ions are always present in the same proportions. Chemical and physical properties of seawater
 Source: researchgate.net
Source: researchgate.net
Two of the most prevalent ions in seawater are chloride and sodium. Sodium and chloride have very long residence times, while calcium (vital for carbonate formation) tends to. Table 26.1 lists the reference values for blood plasma, cerebrospinal fluid (csf), and urine for the six ions addressed in this section.
 Source: slideplayer.com
Source: slideplayer.com
The six most abundant ions of seawater are chloride (cl −), sodium (na +), sulfate (so 2 4 −), magnesium (mg 2+), calcium (ca 2+), and potassium (k +). Animal cells—which lack cell walls—swell and burst if there is a continuous net uptake of water, or shrivel and die if there is a substantial. They come from the atmosphere and dissolves in seawater at the sea surface.
 Source: link.springer.com
Source: link.springer.com
How resource expenditure can be contained to a realistic budget which ions comprise about 85% of the solutes in seawater? Together, they make up around 85 percent of all dissolved ions in the ocean. Principle dissolved ions in seawater 19 3.
 Source: researchgate.net
Source: researchgate.net
Seawater contains at least a little of almost everything but the most of the _____ are _____ 85% sodium and chloride account for about ___% of the ions in seawater Differences like these are due to the varying residence times of seawater solutes; Sodium and chloride have very long residence times, while calcium (vital for carbonate formation) tends to.
 Source: europepmc.org
Source: europepmc.org
*although ocean salinities vary from one part of the ocean to another, forchhammer discovered that salt ions are always present in the same proportions. Together, they make up around 85 percent of all dissolved ions in the ocean. Together, they make up around 85 percent of all dissolved ions in the ocean.
 Source: sciencedirect.com
Source: sciencedirect.com
Salinity is generally low at the equator and at. *although ocean salinities vary from one part of the ocean to another, forchhammer discovered that salt ions are always present in the same proportions. The concentration of salt in seawater (salinity) varies with temperature, evaporation, and precipitation.
 Source: sfamjournals.onlinelibrary.wiley.com
Source: sfamjournals.onlinelibrary.wiley.com
Assume that each of the ions in the nacl solution has the same effect on the freezing point of water as a nonelectrolyte molecule, and determine the freezing temperature the solution (which is approximately. Magnesium and sulfate make up another 10 percent of the total. Over time, the rates of water uptake and loss must balance.
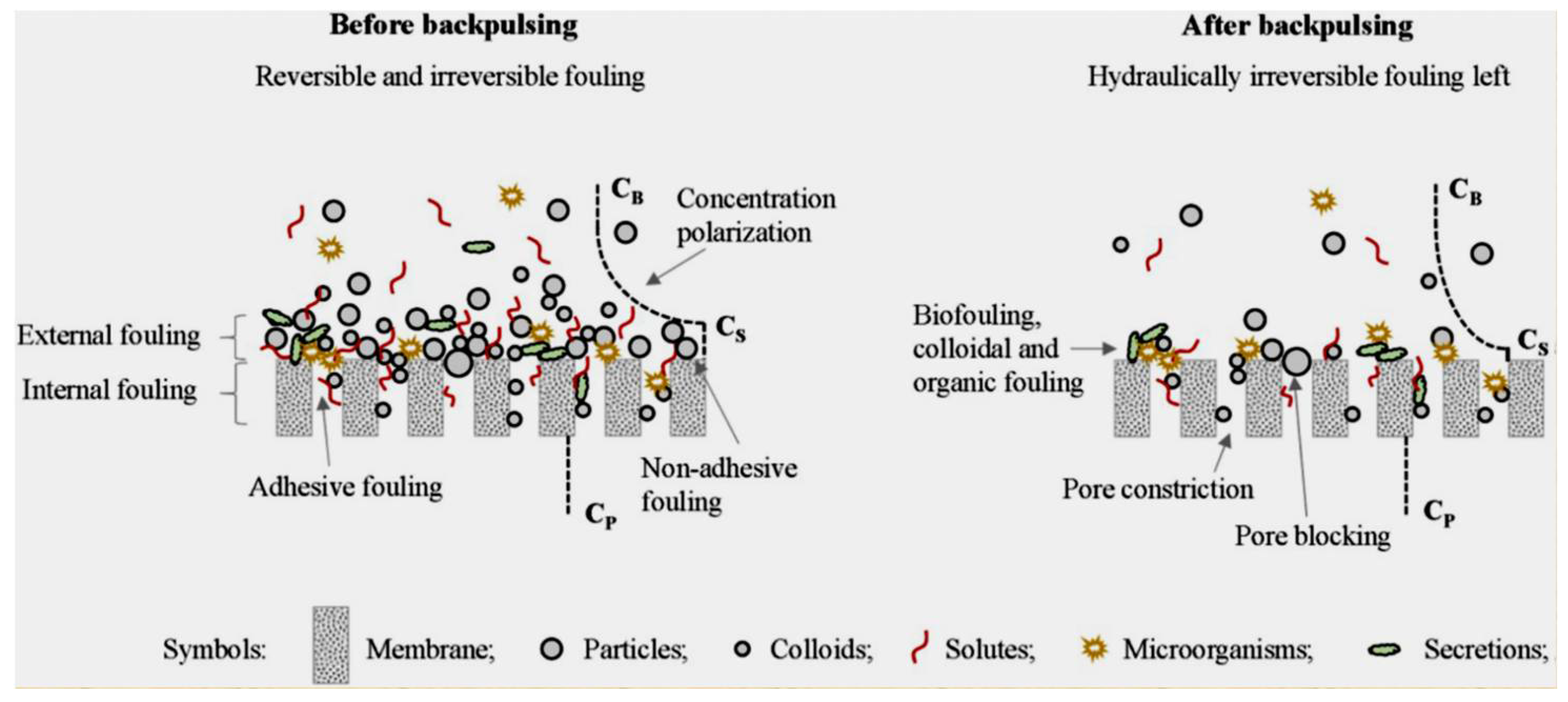 Source: mdpi.com
Source: mdpi.com
Animal cells—which lack cell walls—swell and burst if there is a continuous net uptake of water, or shrivel and die if there is a substantial. A solvent is a major component in a solution. Salinity is generally low at the equator and at.
Also Read :





