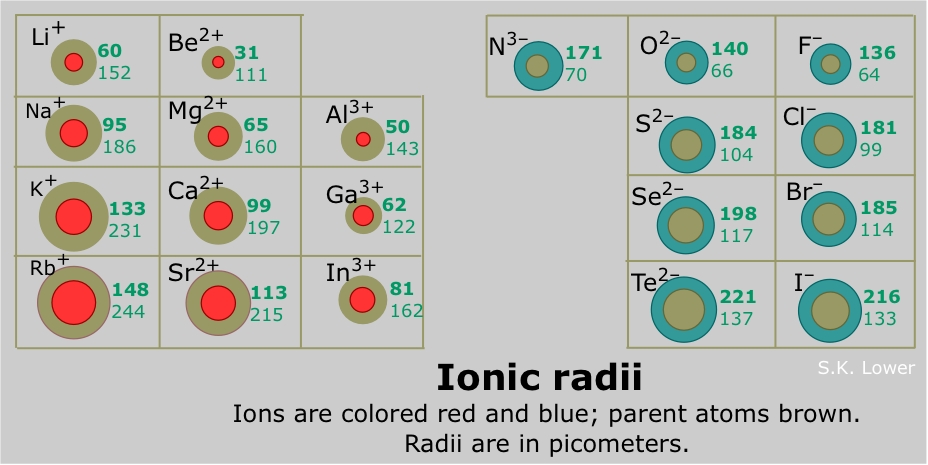
Which element’s ionic radius is smaller? B) s 2− → anion with 18 e −.
Which Ion Has The Largest Radius. Thus, helium is the smallest element, and francium is the largest. Among the followin four ions of the elements of group three of the periodic table ac (3+) has the largest ionic radius. Si, p, s, or al. Ca2+ would have the smallest ionic radius because calcium has a positive charge, and because this ion is a cation, cations will have the smallest radius.
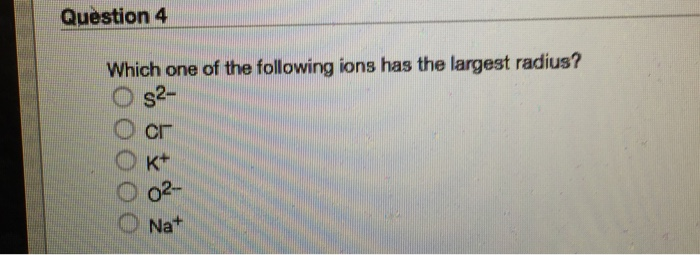 Solved Question 4 Which One Of The Following Ions Has The | Chegg.com From chegg.com
Solved Question 4 Which One Of The Following Ions Has The | Chegg.com From chegg.com
Related Post Solved Question 4 Which One Of The Following Ions Has The | Chegg.com :
Among the followin four ions of the elements of group three of the periodic table ac (3+) has the largest ionic radius. Which ion has the largest radius? Which element has the highest 4th ionization energy? C) na + → cation with 10 e −.
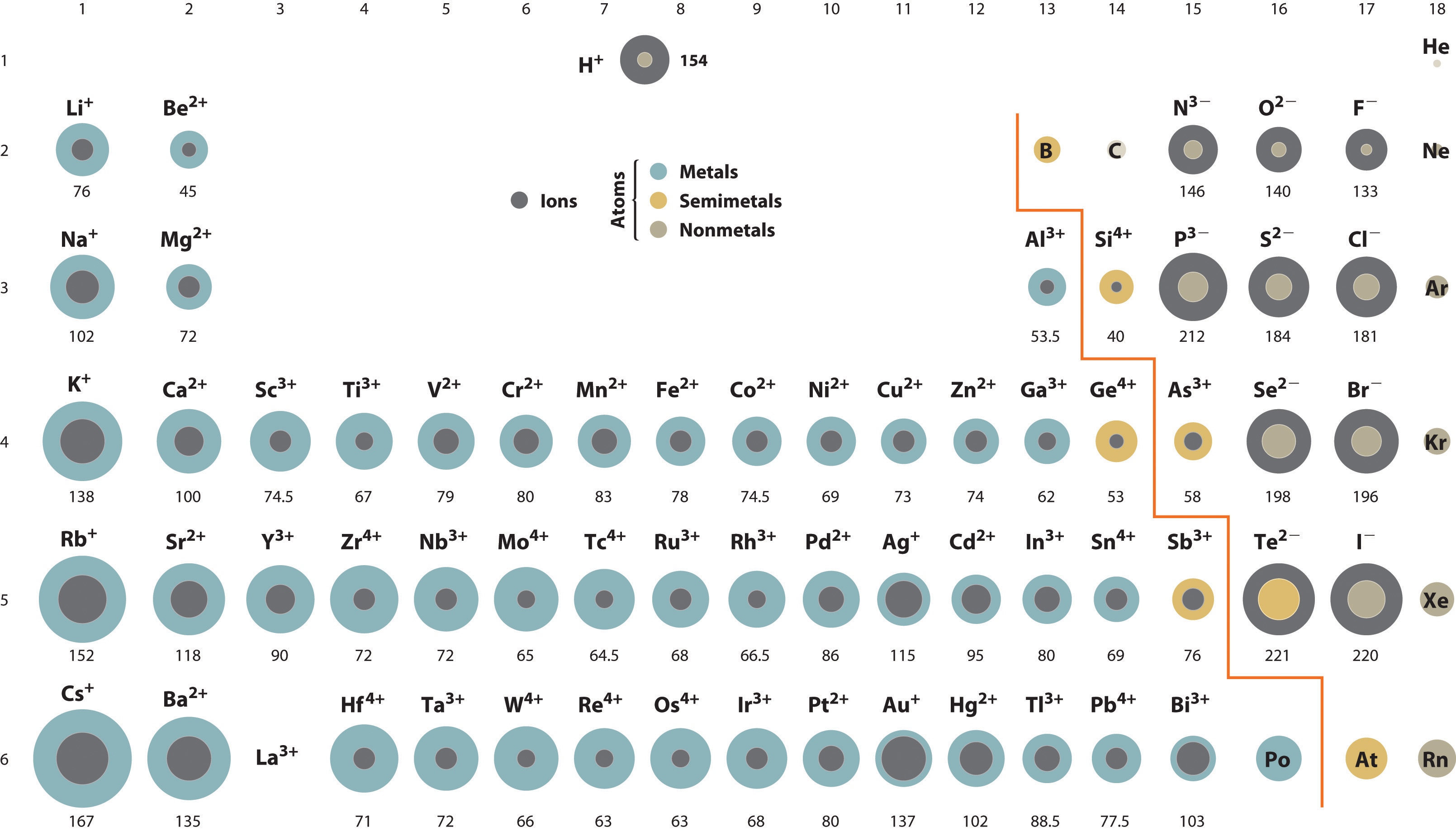
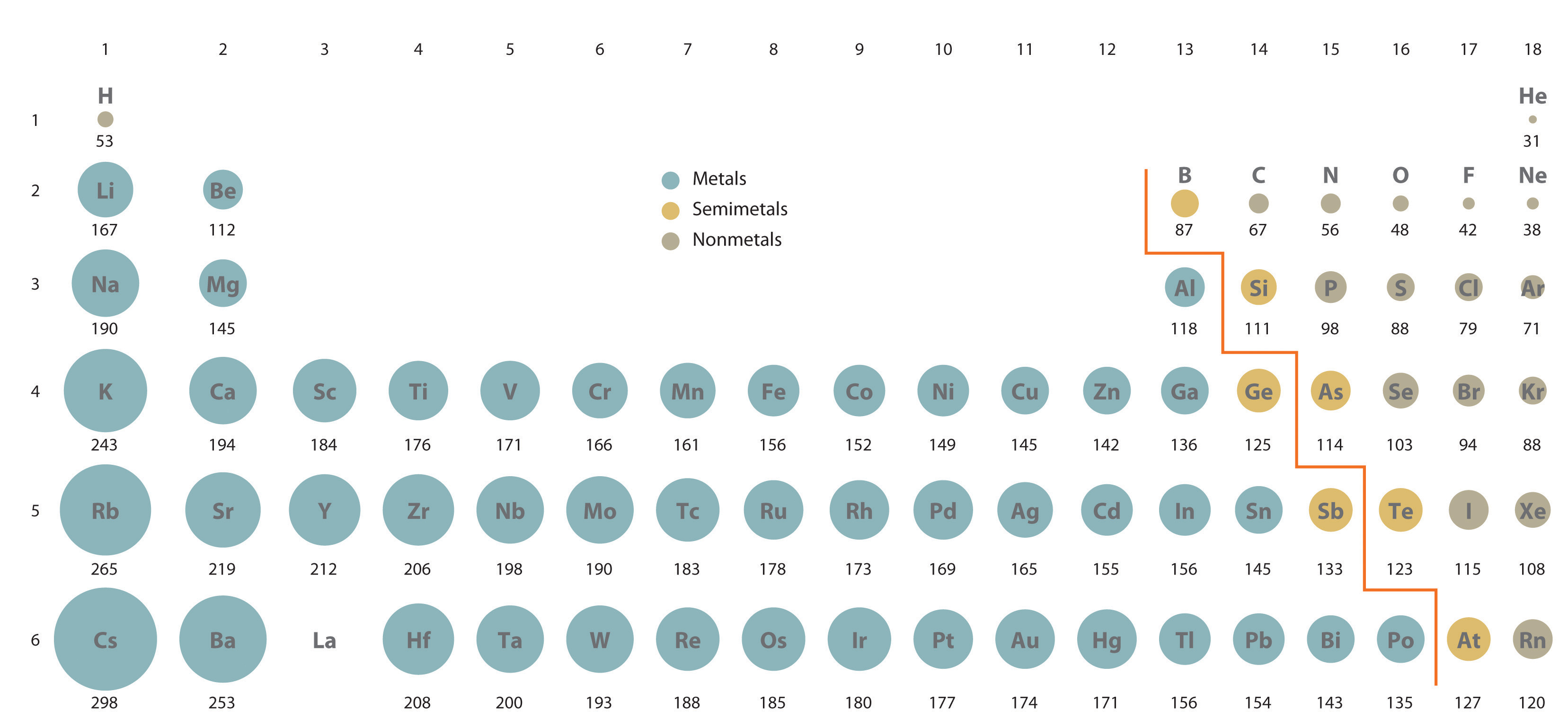
The periodic table is accompanied by many trends.
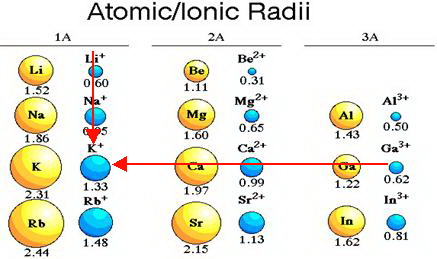
Sc belongs to period 4 , y belongs to period 5, la belongs to period 6 and ac belongs to period 7 of the periodic table. From left to right across the periodic table, atoms get smaller (apart from noble gases) 2. So therefore, elements with a positive charge, will have a smaller ionic radius. Sodium is much more apt to exist as a positive ion than is chlorine. K+ has a larger atomic radius than na+. Which has a larger radius f or f?
 Source: saylordotorg.github.io
Source: saylordotorg.github.io
As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. C) na + → cation with 10 e −. K+ has the largest radius.
 Source: clutchprep.com
Source: clutchprep.com
Thus, the ionic radius of cs+ is the largest. From left to right across the periodic table, atoms get smaller (apart from noble gases) 2. Down any group in the periodic table, atoms get larger.
 Source: chegg.com
Source: chegg.com
Which ion has the largest radius? They increase from top to bottom and from right to left in the periodic table. This is because as one electron is removed from the valence shell of the atom, the effective nuclear charge on the valence shell decreases.
 Source: clutchprep.com
Source: clutchprep.com
K+ has the largest radius. Ca2+ would have the smallest ionic radius because calcium has a positive charge, and because this ion is a cation, cations will have the smallest radius. Positive ions are much smaller than.
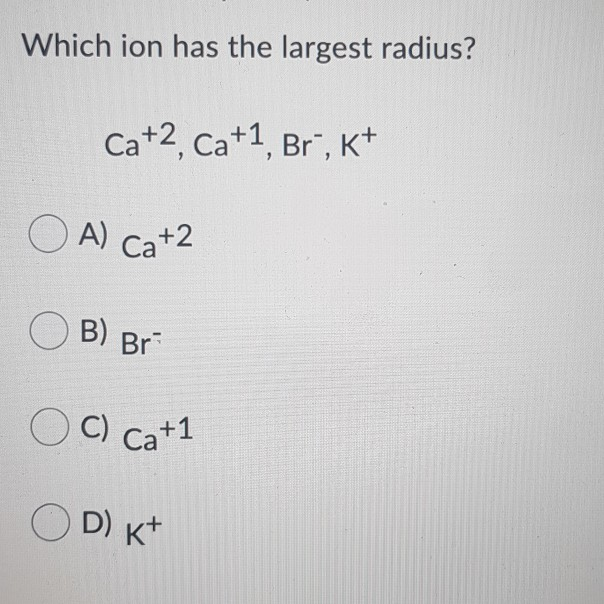 Source: chegg.com
Source: chegg.com
Sodium is much more apt to exist as a positive ion than is chlorine. Down any group in the periodic table, atoms get larger. D) f − → anion with 10 e −.
 Source: sciencenotes.org
Source: sciencenotes.org
Atomic radii vary in a predictable way across the periodic table. Franciumatomic radii vary in a predictable way across the periodic table. Which of the following ions and atoms has the largest radius?
 Source: toppr.com
Source: toppr.com
Which of the following ions has the largest radius? So therefore, elements with a positive charge, will have a smaller ionic radius. Which ion has the largest radius?
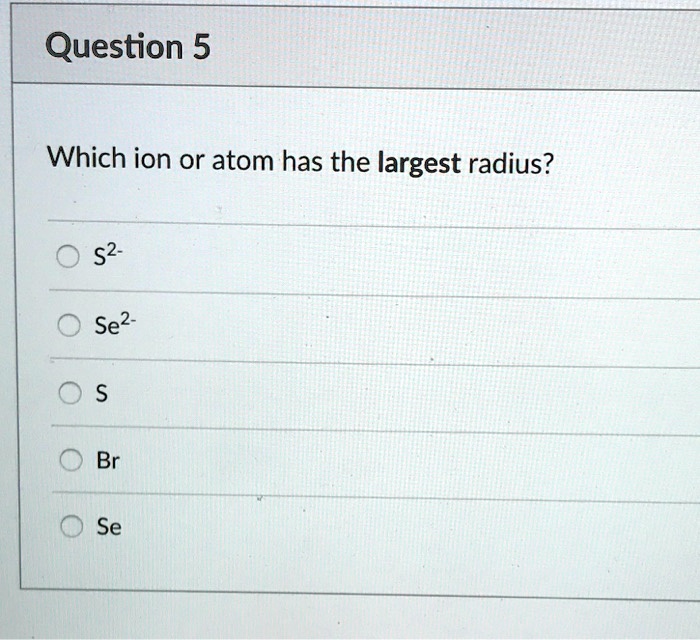 Source: itprospt.com
Source: itprospt.com
O2− is larger than o because o is neutral but o2− has a charge 2−. Thus, helium is the smallest element, and francium is the largest. Let us help you simplify your studying.
 Source: socratic.org
Source: socratic.org
Which atom or ion has the smallest radius? This can be explained by the following logic. Ions with largest radius (identifying) consider these facts:
 Source: clutchprep.com
Source: clutchprep.com
If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back! They increase from top to bottom and from right to left in the periodic table. Which of the following has the largest radius k na+ na k+?
 Source: chegg.com
Source: chegg.com
Among the followin four ions of the elements of group three of the periodic table ac (3+) has the largest ionic radius. As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Positive ions are much smaller than.
 Source: chegg.com
Source: chegg.com
Ca2+ would have the smallest ionic radius because calcium has a positive charge, and because this ion is a cation, cations will have the smallest radius. Which atom or ion has the smallest radius? Franciumatomic radii vary in a predictable way across the periodic table.
 Source: slideplayer.com
Source: slideplayer.com
From left to right across the periodic table, atoms get smaller (apart from noble gases) 2. Hence, k is larger than k+. If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back!
 Source: socratic.org
Source: socratic.org
Which of the following ions and atoms has the largest radius? Check answer and solution for above question fr K (potassium) polar covelant bond will form between which two atoms as shown in the following.
 Source: chegg.com
Source: chegg.com
Thus, the ion with the largest radius is closest to the lower left corner of the periodic table, and that is the k+ ion. They increase from top to bottom and from right to left in the periodic table. K+ has a larger atomic radius than na+.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Which ion is greatest radius in the following? The cation of k+ is formed when k loses this outermost single electron to attain “electronic configuration” of: A) cl − → anion with 18 e −.
 Source: chegg.com
Source: chegg.com
Check answer and solution for above question fr Which element has the highest 4th ionization energy? Which ion has the smallest radius quizlet?
 Source: toppr.com
Source: toppr.com
Which of the following ions and atoms has the largest radius? Thus, the correct option is (a) cs+. D) f − → anion with 10 e −.
 Source: slideplayer.com
Source: slideplayer.com
C) na + → cation with 10 e −. Thus, the ion with the largest radius is closest to the lower left corner of. Z is smaller, and (ii) it is trinegative rather than singly.
 Source: slideserve.com
Source: slideserve.com
Sc belongs to period 4 , y belongs to period 5, la belongs to period 6 and ac belongs to period 7 of the periodic table. Down any group in the periodic table, atoms get larger. That is, all three ions contain 18 electrons but have different nuclear charges.
Also Read :





