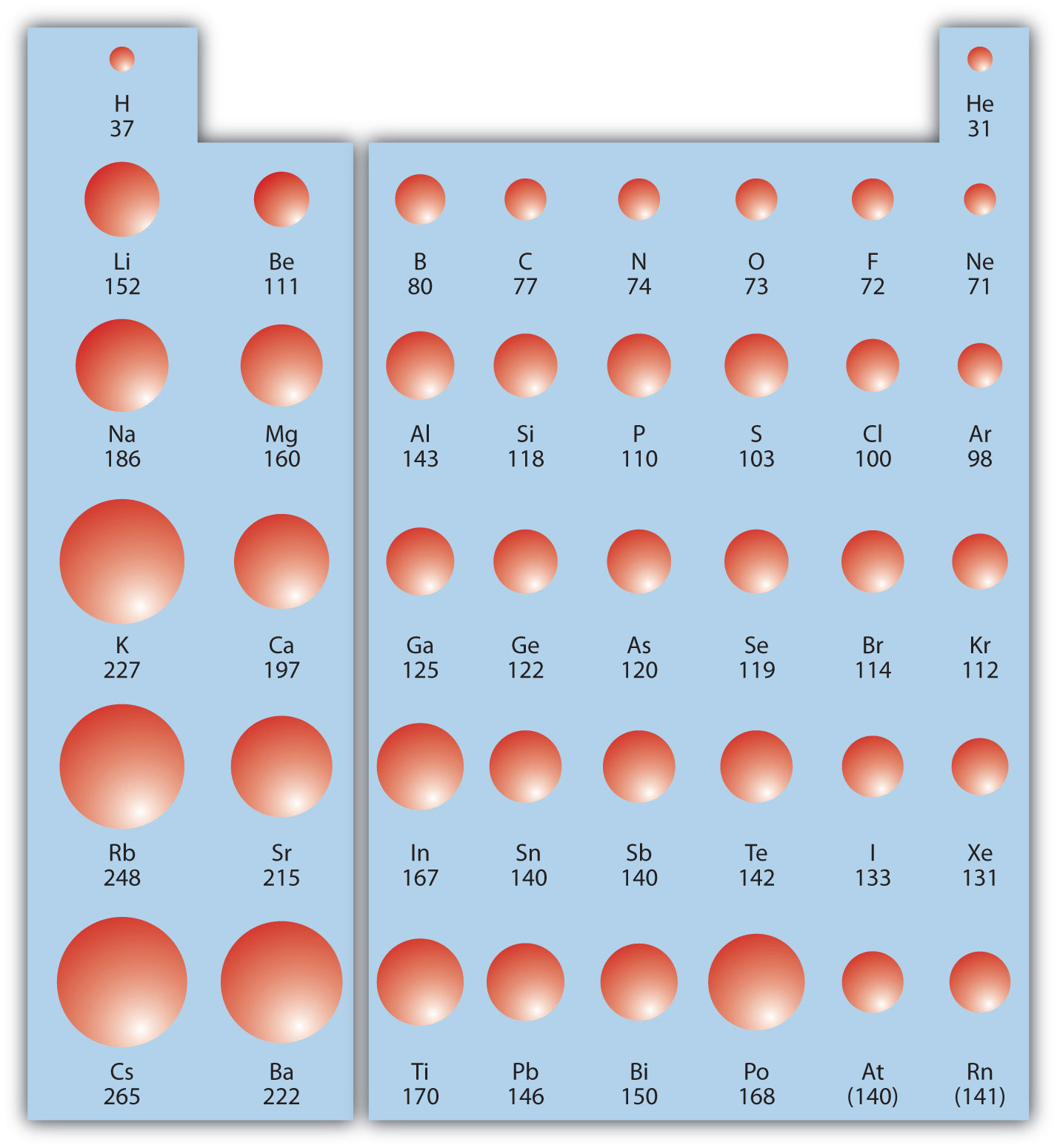
Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius at about 260 pm. (ii) the element li has the smallest atom (atomic radius 152 pm) whereas the element cs has the largest atom (atomic radius 262 pm).
Which Has The Largest Atomic Radius. The atoms have less electrons. The atom with the largest atomic radius is n a which is located in the group 1. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). Of the elements aluminum, al, magnesium, mg, silicon, si, and sodium, na, which has the smallest atomic radius?
 Which One Of The Following Atoms Has The L… | Clutch Prep From clutchprep.com
Which One Of The Following Atoms Has The L… | Clutch Prep From clutchprep.com
Related Post Which One Of The Following Atoms Has The L… | Clutch Prep :
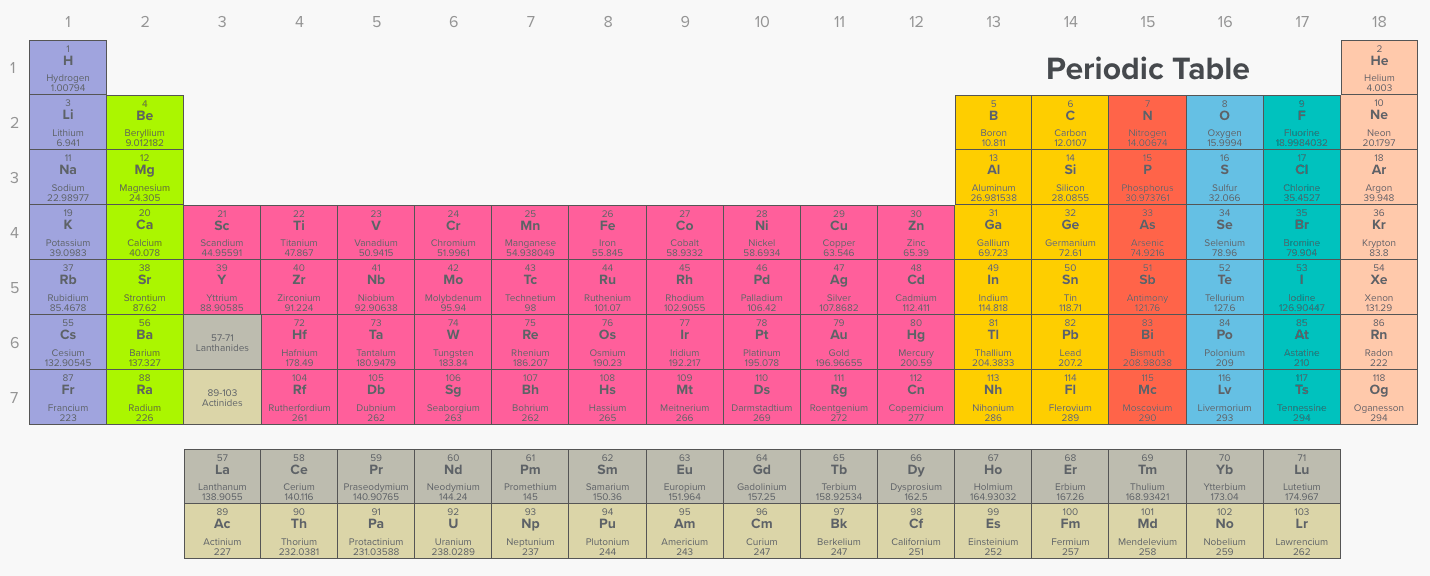
As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Atomic radii vary in a predictable way across the periodic table. Which one of the following elements has the largest atomic radius? Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter).
Which one of the following elements has the largest atomic radius?
The third period will have larger atomic radius than the second period since the third period lies below the second period. See the answer see the answer done loading. Hydrogen (h) has the smallest average atomic radius at about 25 pm, while caesium (cs) has the largest average radius at about 260 pm. As a result, sulfur has the largest atomic radius out of the possible options. What is the size of isoelectronic ions? (ii) the element li has the smallest atom (atomic radius 152 pm) whereas the element cs has the largest atom (atomic radius 262 pm).
 Source: socratic.org
Source: socratic.org
Moreover, n a lies to the left of the periodic table and c l lies to the right of the periodic table. Which has a bigger radius? As you move across the periodic table atoms tend to get smaller because, ______________.
 Source: blog.prepscholar.com
Source: blog.prepscholar.com
Which has a bigger radius? (ii) the element li has the smallest atom (atomic radius 152 pm) whereas the element cs has the largest atom (atomic radius 262 pm). They increase from top to bottom and from right to left in the periodic table.
 Source: clutchprep.com
Source: clutchprep.com
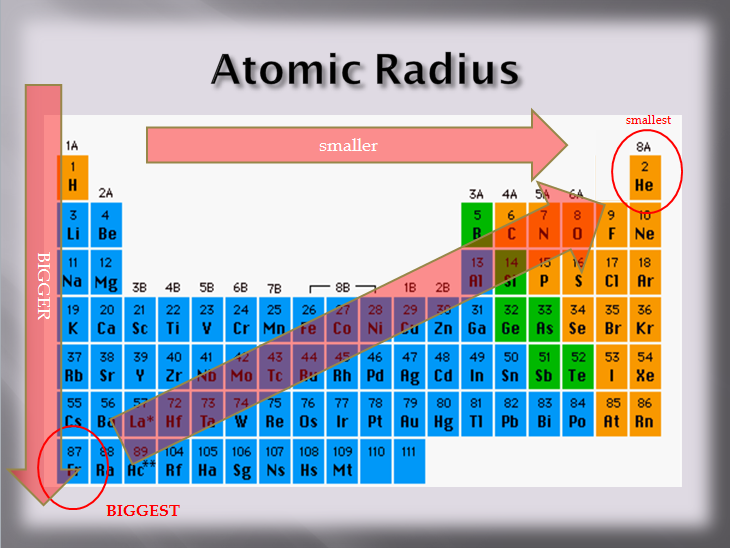
Thus, helium is the smallest element, and francium is the largest. Therefore, there is a greater nuclear attraction. The atoms have less electrons.
 Source: quizlet.com
Source: quizlet.com
Thus, the ion with the largest radius is closest to the lower left corner of the periodic table, and that is the k+ ion. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). From top to bottom in a group, orbitals corresponding to higher.
 Source: study.com
Source: study.com
Barium has the highest atomic number, so barium has the largest atomic radius because atomic radii increase down a group. Thus, helium is the smallest element, and francium is the largest. 1 1 n a has the largest size among these because according to the trend, the atomic radius decreases as we move from left to right in a period of the periodic table.
 Source: sciencenotes.org
Source: sciencenotes.org
Since potassium is located at the start of period 3, and bromine at the end of the same period, potassium will have a larger atomic radius than bromine, and thus the largest atomic radius of the four given atoms. (iii) from this arrangement, we find that the atomic size (or radius) increases down the group. The atoms have more mass.
 Source: angelo.edu
Source: angelo.edu
Atomic radii vary in a predictable way across the periodic table. Because sulfur is to the left of chlorine on the periodic table, it will have a larger atomic radius. Because k+ has the greatest nuclear charge (z = 19), its radius is smallest, and s2− with z = 16 has the largest radius.
 Source: brainly.com
Source: brainly.com
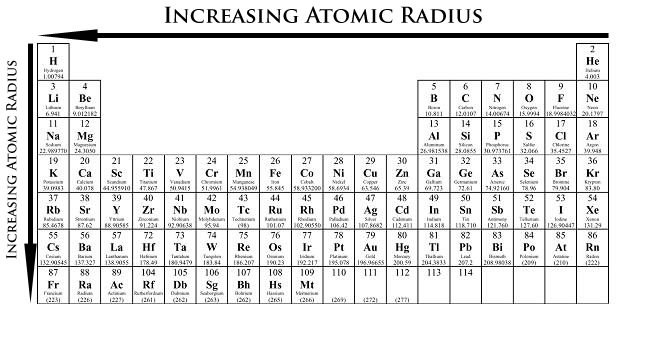
Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). The atomic number of elements increases which means the number of protons and electrons in the atom increases. Trends in the atomic radius showing that moving down the periodic table, size increases, whereas moving across the periodic table from left to right, size decreases.
 Source: youtube.com
Source: youtube.com
Moreover, n a lies to the left of the periodic table and c l lies to the right of the periodic table. How do you know which atom has a larger radius? Franciumatomic radii vary in a predictable way across the periodic table.
 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
See the answer see the answer done loading. Which atom has the largest atomic radius? As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period.

Thus, helium is the smallest element, and francium is the largest. Thus, helium is the smallest element, and francium is the largest. Therefore, rubidium has the largest atomic radius whereas helium has the smallest.

The third period will have larger atomic radius than the second period since the third period lies below the second period. Thus, helium is the smallest element, and francium is the largest. The third period will have larger atomic radius than the second period since the third period lies below the second period.
 Source: sawaal.com
Source: sawaal.com
Thus, helium is the smallest element, and francium is the largest. For atoms or ions that are isoelectronic, the number of protons determines the size. (iii) from this arrangement, we find that the atomic size (or radius) increases down the group.
 Source: socratic.org
Source: socratic.org
Atomic radii vary in a predictable way across the periodic table. Because sulfur is to the left of chlorine on the periodic table, it will have a larger atomic radius. This problem has been solved!
 Source: angelo.edu
Source: angelo.edu
Because k+ has the greatest nuclear charge (z = 19), its radius is smallest, and s2− with z = 16 has the largest radius. 1) br 2) fe 3) ga 4) rb 5) k. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter).
 Source: clutchprep.com
Source: clutchprep.com
Because k+ has the greatest nuclear charge (z = 19), its radius is smallest, and s2− with z = 16 has the largest radius. As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Because sulfur is to the left of chlorine on the periodic table, it will have a larger atomic radius.
 Source: nicepng.com
Source: nicepng.com
Atomic radii vary in a predictable way across the periodic table. (iii) from this arrangement, we find that the atomic size (or radius) increases down the group. The atom with the largest atomic radius is n a which is located in the group 1.
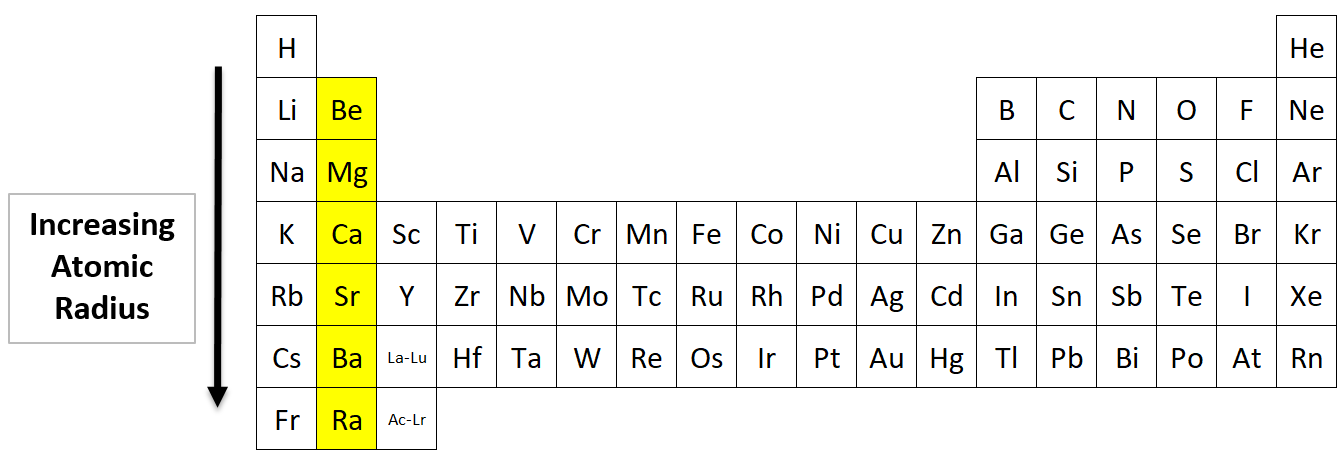 Source: nemoquiz.com
Source: nemoquiz.com
Franciumatomic radii vary in a predictable way across the periodic table. As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. 1 1 n a has the largest size among these because according to the trend, the atomic radius decreases as we move from left to right in a period of the periodic table.
 Source: clutchprep.com
Source: clutchprep.com
Which has a bigger radius? Since potassium is located at the start of period 3, and bromine at the end of the same period, potassium will have a larger atomic radius than bromine, and thus the largest atomic radius of the four given atoms. In which of the following atoms is the 2s orbital closest to the nucleus?

Atomic radii vary in a predictable way across the periodic table. The atoms have more mass. What is the size of isoelectronic ions?
Also Read :





