Ad libitum [116k] 1 year ago. Investigations were carried out in a science lab to explore the topic of chemical and physical changes.
Which Event Always Involves A Chemical Change. Ad libitum [116k] 1 year ago. When burning the wood it doesn�t come back normal. Making a pancake from batter is an example of a change. Expert answered| l_e_v_e_r |points 47|.
 ⚗️Which Event Always Involves A Chemical Change A Boiling B Melting C Conducting D Burning - Brainly.com From brainly.com
⚗️Which Event Always Involves A Chemical Change A Boiling B Melting C Conducting D Burning - Brainly.com From brainly.com
Related Post ⚗️Which Event Always Involves A Chemical Change A Boiling B Melting C Conducting D Burning - Brainly.com :
Burning because example wood and fire. Log in for more information. Investigations were carried out in a science lab to explore the topic of chemical and physical changes. The chemical properties of a substance change, but its physical properties remain the same.
The reappearance of salt is evidence that the change was reversible by a physical change, so it could not be a chemical change.
Burning involves combustion which is done in the presence of oxygen and results in oxides the rest are just state changes. Burning making a pancake from batter is an example of an change. Log in for more information. Which event always involves a chemical change? Burning because example wood and fire. I also took it on e2020.
 Source: chegg.com
Source: chegg.com
I also took it on e2020. Salt to 100 ml warm water and stir until most or all of the salt is no longer. Expert answered| l_e_v_e_r |points 47|.
Burning involves combustion which is done in the presence of oxygen and results in oxides the rest are just state changes. In a science demonstration, a teacher mixed zinc (zn) with hydrogen chloride (hcl) in a flask and quickly attached a balloon over the mouth of the flask. Heat the salt solution on a burner until only a white solid remains.
 Source: eventlooking.com
Source: eventlooking.com
Which event always involves a chemical change ? The chemical properties of a substance change, but its physical properties remain the same. Salt to 100 ml warm water and stir until most or all of the salt is no longer.
 Source: eventlooking.com
Source: eventlooking.com
The reappearance of salt is evidence that the change was reversible by a physical change, so it could not be a chemical change. The correct answer to the question is burning. In a science demonstration, a teacher mixed zinc (zn) with hydrogen chloride (hcl) in a flask and quickly attached a balloon over the mouth of the flask.
 Source: chegg.com
Source: chegg.com
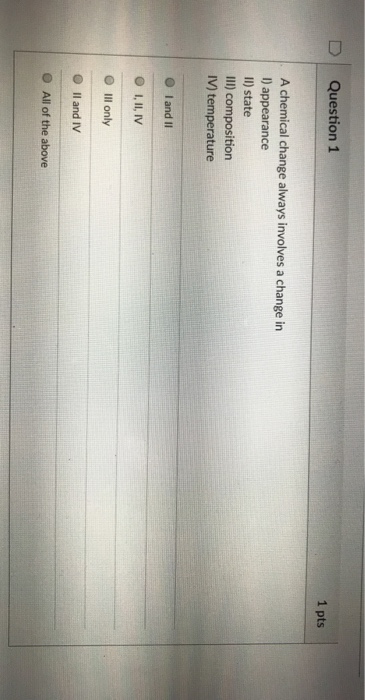
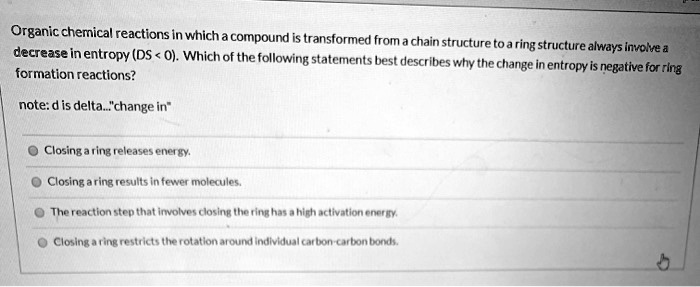
Which event always involves a chemical change? Which event always involves a chemical change? Some properties of a substance change, but its identity remains the same.
 Source: slideplayer.com
Source: slideplayer.com
The corrosion is from oxidation chemical reaction between the metal and oxygen, water, and carbon dioxide in the air. Burning making a pancake from batter is an example of an change. Hence, the correct answer is burning.

A chemical change always involves one or more. Burning making a pancake from batter is an example of an change. I also took it on e2020.
 Source:
Source:
In a science demonstration, a teacher mixed zinc (zn) with hydrogen chloride (hcl) in a flask and quickly attached a balloon over the mouth of the flask. Heat the salt solution on a burner until only a white solid remains. Boiling, melting and conducting are physical changes.
 Source: brainly.com
Source: brainly.com
An example of a chemical change is the reaction between sodium and water to produce sodium hydroxide and hydrogen. I also took it on e2020. The chemical properties of a substance change, but its physical properties remain the same.
 Source: view.genial.ly
Source: view.genial.ly
Burning because example wood and fire. The correct answer to the question is burning. In a science demonstration, a teacher mixed zinc (zn) with hydrogen chloride (hcl) in a flask and quickly attached a balloon over the mouth of the flask.
 Source: slideplayer.com
Source: slideplayer.com
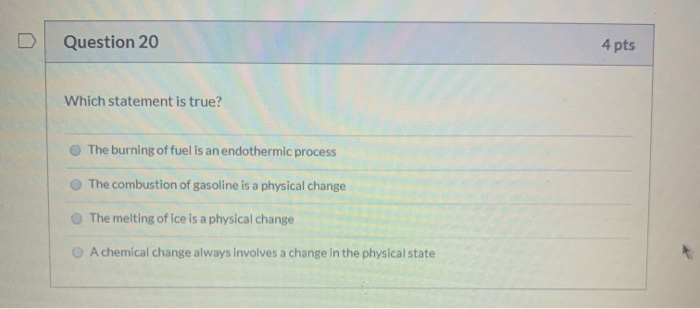
Burning always involves a chemical change. Which event always involves a chemical change ? Burning involves combustion which is done in the presence of oxygen and results in oxides the rest are just state changes.
 Source: thoughtco.com
Source: thoughtco.com
Which event always involves a chemical change? In transpiration, because some of its properties change, water undergoes a physical change but keeps its identity. Ad libitum [116k] 1 year ago.
 Source: slideplayer.com
Source: slideplayer.com
In transpiration, because some of its properties change, water undergoes a physical change but keeps its identity. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products are generated. An example of a chemical change is the reaction between sodium and water to produce sodium hydroxide and hydrogen.
 Source: slideserve.com
Source: slideserve.com
Rust is the term used to describe this process when it happens to iron instead of other metals. Salt to 100 ml warm water and stir until most or all of the salt is no longer visible. Log in for more information.
 Source: brainly.com
Source: brainly.com
The chemical properties of a substance change, but its physical properties remain the same. Some properties of a substance change, but its identity remains the same. Out of the four options given in the question, only burning is a chemical change.
 Source: slideplayer.com
Source: slideplayer.com
Which event always involves a chemical change? Both the identity and the properties of a substance change. Burning always involves a chemical change.
 Source: eventlooking.com
Source: eventlooking.com
In a science demonstration, a teacher mixed zinc (zn) with hydrogen chloride (hcl) in a flask and quickly attached a balloon over the mouth of the flask. Which event always involves a chemical change ? Boiling, melting and conducting are physical changes.
 Source: eventlooking.com
Source: eventlooking.com
Burning because example wood and fire. The chemical properties of a substance change, but its physical properties remain the same. In photosynthesis, because its identity.
 Source: slidetodoc.com
Source: slidetodoc.com
Investigations were carried out in a science lab to explore the topic of chemical and physical changes. Log in for more information. Burning, cooking, rusting, and rotting.
 Source: slideplayer.com
Source: slideplayer.com
You might be interested in. Investigations were carried out in a science lab to explore the topic of chemical and physical changes. In this type of change, a new compound is formed which have different properties as compared to the reactants.
Also Read :