Ab → a + b. C 2 h 5 oh + 3o 2 → 2co 2 + 3h 2 o.
Which Equation Represents A Decomposition Reaction. When propane is reacted in the presence of oxygen gas, the products of this combustion reaction are: Ab → a + b. 1.what is a decomposition reaction? (1) caco 3(s) ==>cao (s) + co 2(g) (2) cu (s) + 2agno 3(aq) ==>2ag (s) + cu(no 3) 2(aq) (3) 2h 2(g) + o 2(g) ==>2h 2 o (l) (4) koh (aq) + hcl (aq) ==>kcl (aq) + h 2 o (l) 1:
 The Equation, Mg_((S))+Cuo_((S))Tomgo_((S))+Cu_((S)) Represents (A) Decomposition Reaction (B) Displacement Reaction ( C) Combination Reaction (D) Double Displacement Reaction (E) Redox Reaction From doubtnut.com
The Equation, Mg_((S))+Cuo_((S))Tomgo_((S))+Cu_((S)) Represents (A) Decomposition Reaction (B) Displacement Reaction ( C) Combination Reaction (D) Double Displacement Reaction (E) Redox Reaction From doubtnut.com
Related Post The Equation, Mg_((S))+Cuo_((S))Tomgo_((S))+Cu_((S)) Represents (A) Decomposition Reaction (B) Displacement Reaction ( C) Combination Reaction (D) Double Displacement Reaction (E) Redox Reaction :
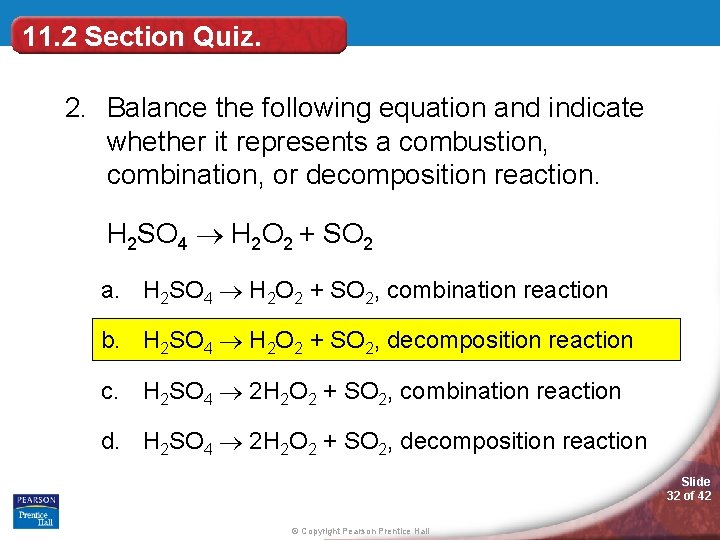
(1) a single replacement reaction (2) a double replacement reaction (3) a complete combustion reaction (4) a decomposition reaction 13. (1) caco 3(s) ==>cao (s) + co 2(g) (2) cu (s) + 2agno 3(aq) ==>2ag (s) + cu(no 3) 2(aq) (3) 2h 2(g) + o 2(g) ==>2h 2 o (l) (4) koh (aq) + hcl (aq) ==>kcl (aq) + h 2 o (l) 1: A decomposition reaction occurs when one reactant breaks down into two or more products. Decomposition reactions are initiated by the addition of energy.
Decomposition reactions are initiated by the addition of energy.
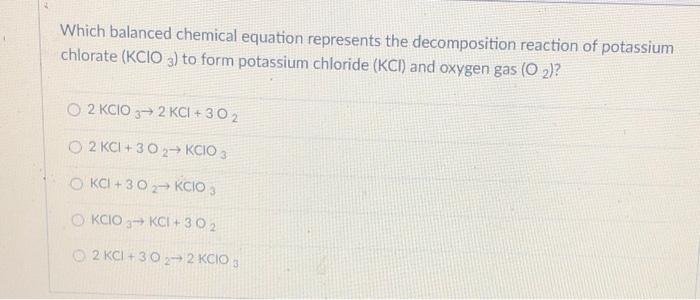
Which chemical equation correctly represents the decomposition reaction that takes place when ammonia breaks down to form hydrogen gas and nitrogen gas? Give reason for the following: Choose the general equation for decomposition reaction: Thermal decomposition of potassium chlorate: Which type of reaction does the above equation show ? When potassium chlorate is heated in the presence of manganese dioxide as a catalyst, it decomposes to give potassium chloride and oxygen.

2nacl c.2k + 2h 2o. The breaking of chemical bonds requires the addition of energy, usually in the form of heat. When a compound is heated, its atoms move about.
 Source: slideplayer.com
Source: slideplayer.com
A decomposition reaction involves the breaking up of a substances into its constituents as follows; Decomp ab==> a + b: (1) caco3(s) ==>cao(s) + co2(g) (2) cu(s) + 2agno3(aq) ==>2ag(s) + cu(no3)2(aq) (3) 2h2(g) + o2(g) ==>2h2o(l) (4).
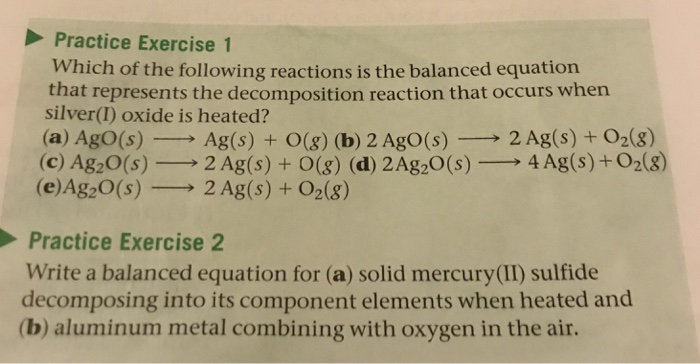
Choose the general equation for decomposition reaction: The reaction equation is as follows: Ab → a + b.
 Source: slideplayer.com
Source: slideplayer.com
Which one of the following chemical equations represents a decomposition reaction? 2hbr + cl 2 → 2hcl + br 2. A decomposition reaction involves the breaking up of a substances into its constituents as follows;
 Source: slideplayer.com
Source: slideplayer.com
2h 2 o→ 2h 2 + o 2 When potassium chlorate is heated in the presence of manganese dioxide as a catalyst, it decomposes to give potassium chloride and oxygen. Nh 3 + h 2 → n 2 b.
 Source: brainly.com
Source: brainly.com
Ab → a + b. What is the general equation for a decomposition reaction? Decomp ab==> a + b:
2.describe the decomposition of hydrogen peroxide, and write a balanced chemical equation for this reaction. An example of an electrolytic decomposition reaction is the electrolysis of water, which can be represented by the following chemical equation: It is represented by the general equation:
 Source: chemistrylearner.com
Source: chemistrylearner.com
Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen, and the breakdown of water to hydrogen and oxygen. (1) caco 3(s) ==>cao (s) + co 2(g) (2) cu (s) + 2agno 3(aq) ==>2ag (s) + cu(no 3) 2(aq) (3) 2h 2(g) + o 2(g) ==>2h 2 o (l) (4) koh (aq) + hcl (aq) ==>kcl (aq) + h 2 o (l) 1: Be sure to include the correct states in your final equations.
 Source: slideplayer.com
Source: slideplayer.com
Na2co3 + co2 + h2o the reaction is a decomposition reaction because one compound, sodium bicarbonate, spontaneously breaks apart into multiple. Na 2 o + co 2 → na 2 co 3. Which of the following reactions is the balanced equation that represents the decomposition reaction that occurs when silver (i) oxide is heated?
 Source: youtube.com
Source: youtube.com
Na 2 co 3 → na 2 o + co 2. When propane is reacted in the presence of oxygen gas, the products of this combustion reaction are: Cl2 + 2nabr ® 2nacl + br2 2nacl ® 2na + cl2 a)single replacement and decomposition b) single replacement and double replacement c) synthesis and decomposition d) synthesis and double replacement which type of chemical reactions are represented by these equations?
 Source: oneclass.com
Source: oneclass.com
(1) a single replacement reaction (2) a double replacement reaction (3) a complete combustion reaction (4) a decomposition reaction 13. Be sure to include the correct states in your final equations. (1) 1 (3) 3 (2) 2 (4) 4 14.
 Source: doubtnut.com
Source: doubtnut.com
When potassium chlorate is heated in the presence of manganese dioxide as a catalyst, it decomposes to give potassium chloride and oxygen. The reaction equation is as follows: A decomposition reaction is a chemical reaction in which some chemical bonds in a compound are broken and simpler substances are formed.
 Source: slideplayer.com
Source: slideplayer.com
If no reaction is expected, write “no reaction.” ans: A) oxidation b) reduction c) corrosion d. Which type of reaction does the above equation show ?
 Source: chegg.com
Source: chegg.com
Answer choices n2 + h2 → nh3 Decomposition reactions are initiated by the addition of energy. Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen, and the breakdown of water to hydrogen and oxygen.
 Source: chegg.com
Source: chegg.com
Review what is a decomposition reaction? A decomposition reaction is the reverse of a synthesis reaction and is a reaction in which a single compound undergoes a reaction that produces two or more simpler substances. Cucl 2 (aq) + pb(s) cu(s) + pbcl 2 (s) net ionic equation:
 Source: youtube.com
Source: youtube.com
A) combination reaction b) decomposition reaction c) displacement reaction d) double displacement reaction answer: 35 which equation represents a decomposition reaction? This can be represented by the general equation:

A decomposition reaction occurs when one reactant breaks down into two or more products. Give reason for the following: Which type of reaction does the above equation show ?
 Source: slidetodoc.com
Source: slidetodoc.com
Ab → a + b. 38.identify the type of chemical reaction represented by this equation. Cucl 2 (aq) + pb(s) cu(s) + pbcl 2 (s) net ionic equation:
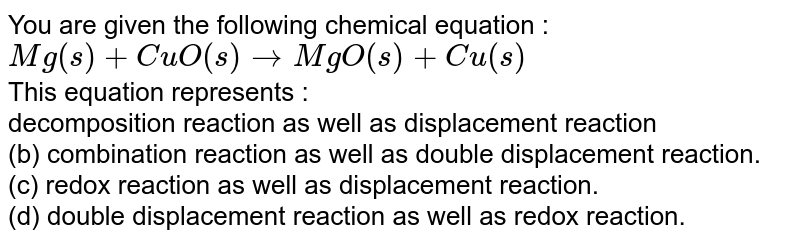 Source: doubtnut.com
Source: doubtnut.com
Which of the following is an example of a combustion reaction? If no reaction is expected, write “no reaction.” ans: (1) caco 3(s) ==>cao (s) + co 2(g) (2) cu (s) + 2agno 3(aq) ==>2ag (s) + cu(no 3) 2(aq) (3) 2h 2(g) + o 2(g) ==>2h 2 o (l) (4) koh (aq) + hcl (aq) ==>kcl (aq) + h 2 o (l) 1:

Nh 3 → n 2 + h 2 _____ 2. A decomposition reaction occurs when one reactant breaks down into two or more products. Na 2 co 3 → na 2 o + co 2.
Also Read :