This type of bond is formed when electrons are shared between atoms. In general, electronegativity differences greater than about 2.0 are considered to be ionic bonds.
Which Element Reacts With Oxygen To Form Ionic Bonds. Get 20% off grade+ yearly subscription → Oxygen does not contain ionic bonds. Electrons are shared and the bonding is covalent. But calcium is a metal and it forms ionic bonds with oxygen.
 Ionic Bonding - Wikipedia From en.wikipedia.org
Ionic Bonding - Wikipedia From en.wikipedia.org
Related Post Ionic Bonding - Wikipedia :
It reacts with all metals except gold and platinum, forming sulfides; If the difference is (1.7) or more, an ionic bond is favoured. How does calcium and oxygen form an ionic bond? A)h2 b)ch4 c)ch3oh d)nh 4cl
To see more answers head over to college study guides.
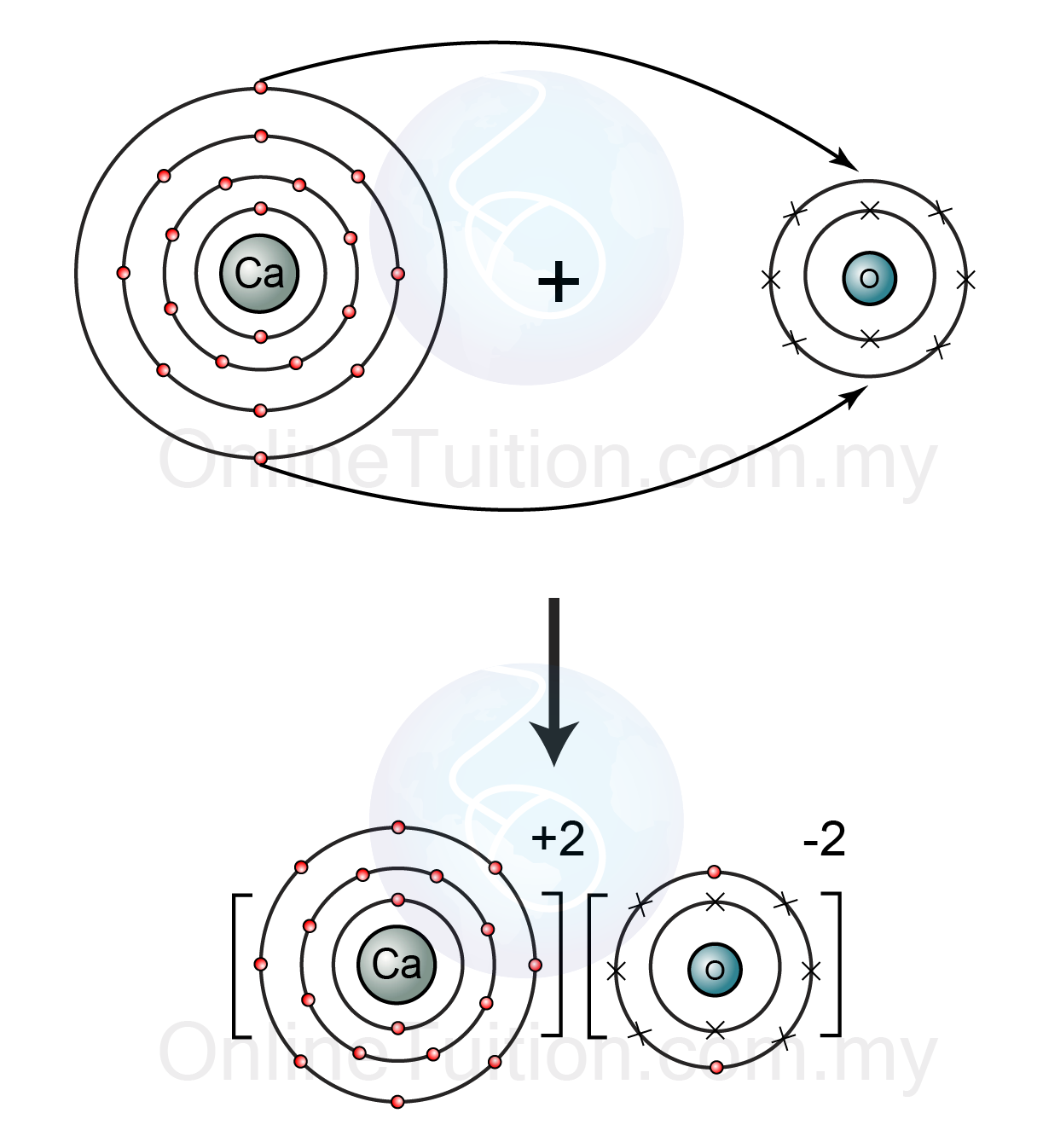
Can you look at magnesium reacting with oxygen? Hope it helps you mark as brainliest In general, electronegativity differences greater than about 2.0 are considered to be ionic bonds. 35 which element reacts with oxygen to form ionic bonds? Calcium loses two electrons to form and these electrons are gained by oxygen, resulting in the formation of. How does calcium and oxygen form an ionic bond?
 Source: youtube.com
Source: youtube.com
Which elements can form ionic bonds? How does calcium and oxygen form an ionic bond? Does oxygen form ionic bonds?
It is soluble in water, reacting to form calcium hydroxide. Can you look at magnesium reacting with oxygen? In general, electronegativity differences greater than about 2.0 are considered to be ionic bonds.
 Source: brainly.com
Source: brainly.com
Since two aluminum ions each with +3 charge will balance the charge on three oxide ions with. As the products of the reaction are at a lower energy level than the reactants, the result is an explosive release of energy and the production of water. The electronegativity of oxygen is 3.5 therefore any of the alkali or alkaline metals will ionically bond with oxygen.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Calcium loses two electrons to form and these electrons are gained by oxygen, resulting in the formation of. Which substance is an ionic solid? Identify the element that fluorine forms an ionic bond with oxygen phosphorus sodium xenon carbon which of the following is an ionic compound mgcl_2 cl_2 scl_2 pcl_5 ch_2o which of the following is a molecular compound?
 Source: numerade.com
Source: numerade.com
How does calcium and oxygen form an ionic bond? But calcium is a metal and it forms ionic bonds with oxygen. Ionic bonds usually occur between metal and nonmetal ions.
 Source: study.com
Source: study.com
Electrons are shared and the bonding is covalent. Get 20% off grade+ yearly subscription → Chemical bonds & formulas after school regents review practice a)calcium b)hydrogen c)chlorine d)nitrogen 22.which element reacts with oxygen to form ionic bonds?
 Source: socratic.org
Source: socratic.org
This type of bond is formed when electrons are shared between atoms. This type of bond is formed when electrons are shared between atoms. In the water molecule, each hydrogen atom shares one.
 Source: slideplayer.com
Source: slideplayer.com
Which element reacts with oxygen to form ionic bonds? In a covalent bond, the atoms bond by sharing electrons. Ionic bonds usually occur between metal and nonmetal ions.
 Source: study.com
Source: study.com
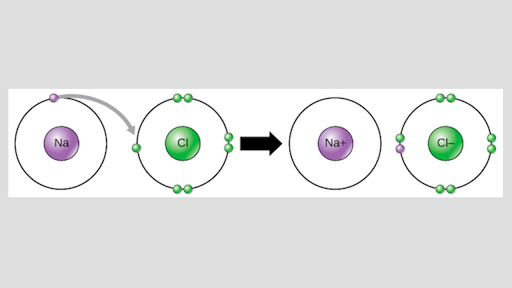
For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. Which pair of elements form an ionic bond with each other? An ionic bond by definition is the kind of chemical bond formed when there is a complete transfer of valence electrons from one atom to another, generating two oppositely charged ions.
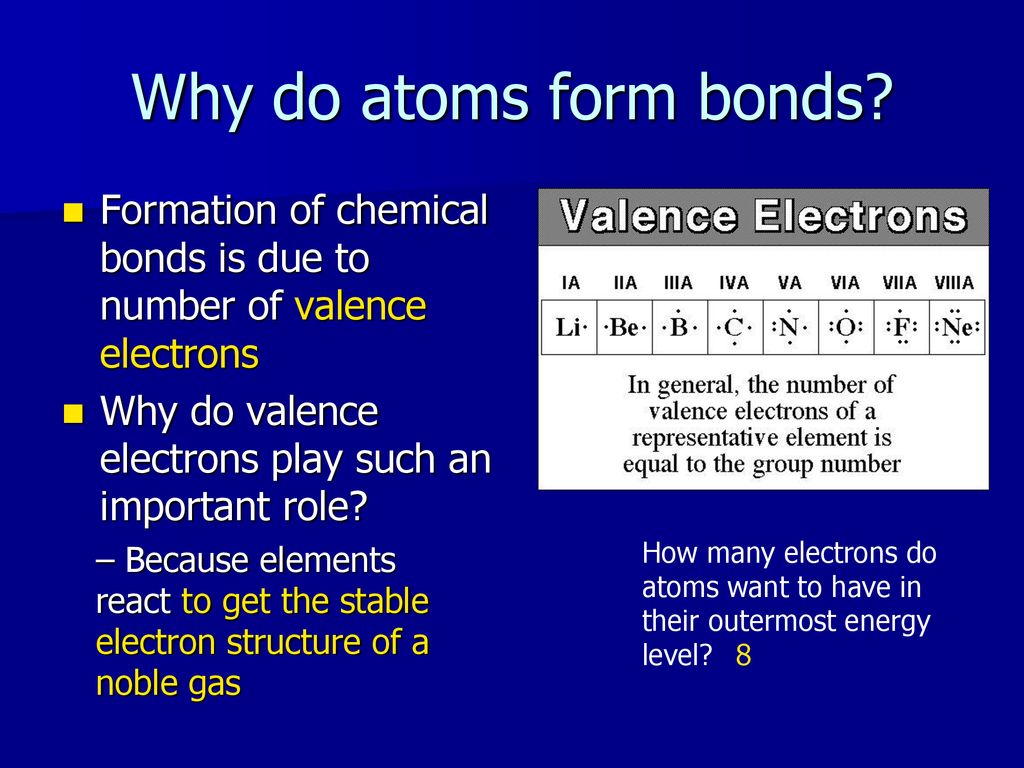 Source: slideplayer.com
Source: slideplayer.com
Figure 1 the ionic bonding in magnesium oxide (mgo). As you may know, each atom contains a set amount of electrons. Hence, the element must be very reactive and must form an ionic bond.

Which pair of elements form an ionic bond with each other? Is sulfur and oxygen polar or nonpolar? 35 which element reacts with oxygen to form ionic bonds?
 Source: indiascreen.ir
Source: indiascreen.ir
The copper oxide can then react with the hydrogen gas to form the copper metal and water. Which element reacts with oxygen to form ionic bonds? A)electrons are shared and the bonding is ionic.
 Source: brainly.in
Source: brainly.in
Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen and hydrogen atoms. Chemical bonds & formulas after school regents review practice a)calcium b)hydrogen c)chlorine d)nitrogen 22.which element reacts with oxygen to form ionic bonds? Answer it is given that the element reacts with oxygen to give a high melting point.
 Source: savemyexams.co.uk
Source: savemyexams.co.uk
Electrons are transferred and the bonding is covalent. In general, electronegativity differences greater than about 2.0 are considered to be ionic bonds. Which element reacts with oxygen to form ionic bonds?

Which element reacts with oxygen to form ionic bonds? In the water molecule, each hydrogen atom shares one. Which element reacts with oxygen to form ionic bonds?
 Source: oneclass.com
Source: oneclass.com
Lithium is in group 1 of the periodic table. This 18 words question was. Is sulfur and oxygen polar or nonpolar?
 Source: chegg.com
Source: chegg.com
Chemical bonds & formulas after school regents review practice a)calcium b)hydrogen c)chlorine d)nitrogen 22.which element reacts with oxygen to form ionic bonds? The copper oxide can then react with the hydrogen gas to form the copper metal and water. Can you look at magnesium reacting with oxygen?
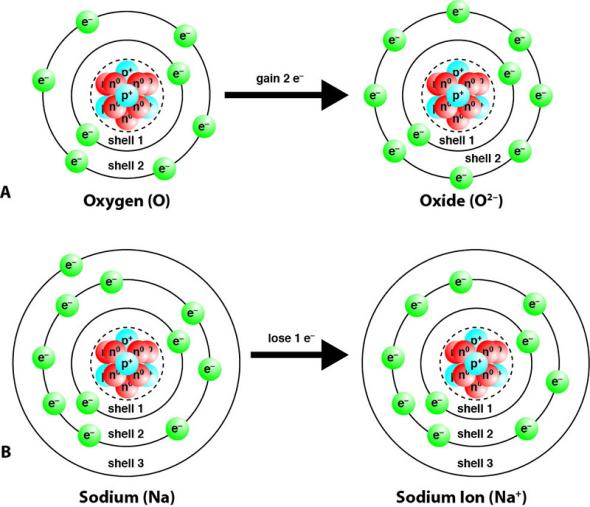
In the water molecule, each hydrogen atom shares one. Hydrogen molecules violently react with oxygen when the existing molecular bonds break and new bonds are formed between oxygen and hydrogen atoms. As you may know, each atom contains a set amount of electrons.
 Source: manoa.hawaii.edu
Source: manoa.hawaii.edu
Identify the element that fluorine forms an ionic bond with oxygen phosphorus sodium xenon carbon which of the following is an ionic compound mgcl_2 cl_2 scl_2 pcl_5 ch_2o which of the following is a molecular compound? Since two aluminum ions each with +3 charge will balance the charge on three oxide ions with. It also forms compounds with several nonmetallic elements.
 Source: docbrown.info
Source: docbrown.info
The copper oxide can then react with the hydrogen gas to form the copper metal and water. It also forms compounds with several nonmetallic elements. Electrons are transferred and the bonding is covalent.
Also Read :