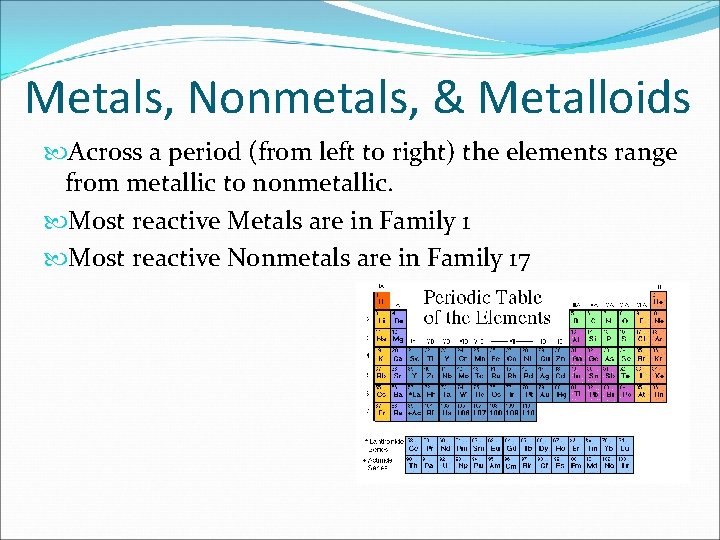
Group 8a (or viiia) of the periodic table are the noble gases or inert gases: Where are the most active metals located most active nonmetals?
Which Element Is The Most Reactive Nonmetal. The most reactive element in period 2 is lithium (atomic number = 3) since it an alkali metal (group 1) electronic configuration = (2,1) thanks. Which nonmetal is the most reactive cl br i? Looking at the same nonmetal group on the periodic table, how does the reactivity of an element in period 2 compare to the reactivity of an element in period 4? The most reactive element from group seven is fluorine which is at the top of that section of the periodic table.
 For Test #6: The Periodic Table - Ppt Download From slideplayer.com
For Test #6: The Periodic Table - Ppt Download From slideplayer.com
Related Post For Test #6: The Periodic Table - Ppt Download :
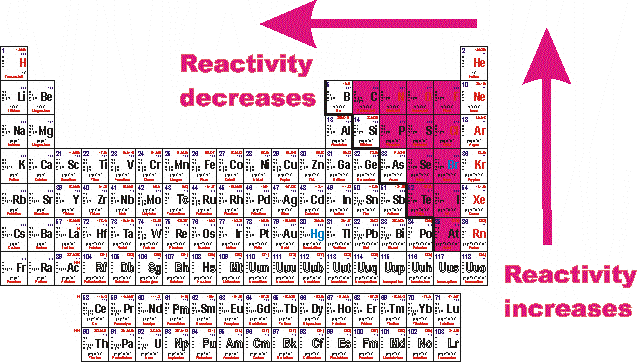
Helium (he), neon (ne), argon (ar), krypton (kr), xenon (xe), and radon (rn). What group of elements are the most reactive nonmetals? The halogen group of elements is the most reactive of the nonmetals. The most reactive metal is francium, the last alkali metal (and most expensive element).
What is the most responsive element?
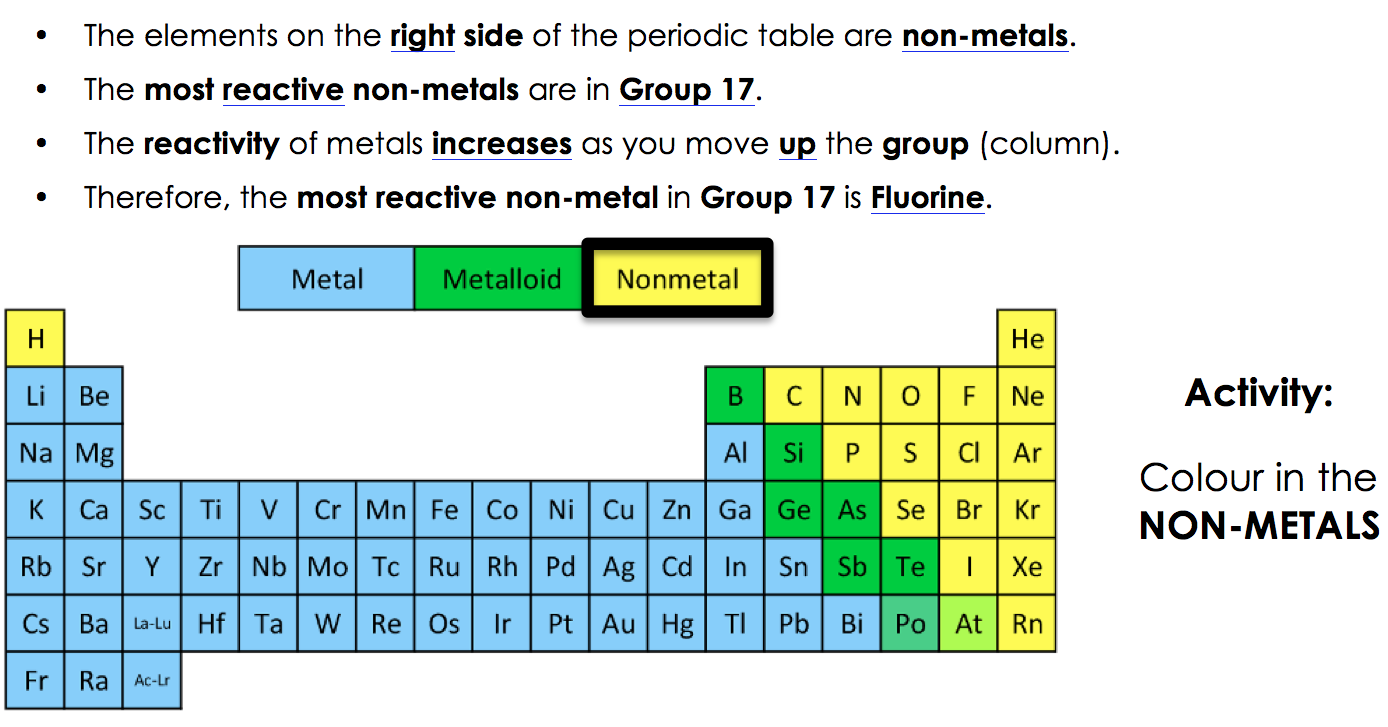
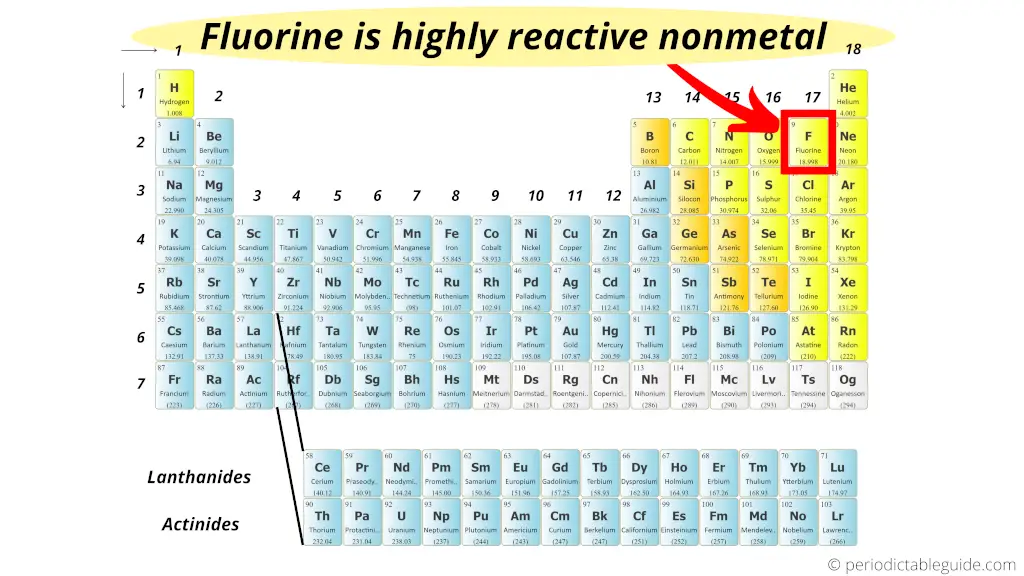
Fluorine is identified as the most reactive nonmetal and the most electronegative element in the periodic table, making it the strongest oxidizing agent. The halogen group of elements is the most reactive of the nonmetals. What element is the most active metal? Due to its strong electro negativity & small size, fluorine has a strong tendency to accept electrons from other atoms or ions. Group 8a — the noble or inert gases. Fluorine is a halogen, which is group 17 on the periodic table, and the halogens are the most reactive nonmetals.
 Source: socratic.org
Source: socratic.org
Due to its strong electro negativity & small size, fluorine has a strong tendency to accept electrons from other atoms or ions. Group 8a (or viiia) of the periodic table are the noble gases or inert gases: The most reactive element is fluorine, the first element in the halogen group.

What group of elements is the most reactive metals and why? The halogen group of elements is the most reactive of the nonmetals. Actually the halogens are the most reactive nonmetals, but we know that as we move down the group, the electronegativity decreases.
 Source: reference.com
Source: reference.com
The most reactive nonmetal is fluorine. The most reactive element in period 2 is lithium (atomic number = 3) since it an alkali metal (group 1) electronic configuration = (2,1) thanks. The reactivity of an element can be predicted based on its location on the periodic table.
 Source: slideplayer.com
Source: slideplayer.com
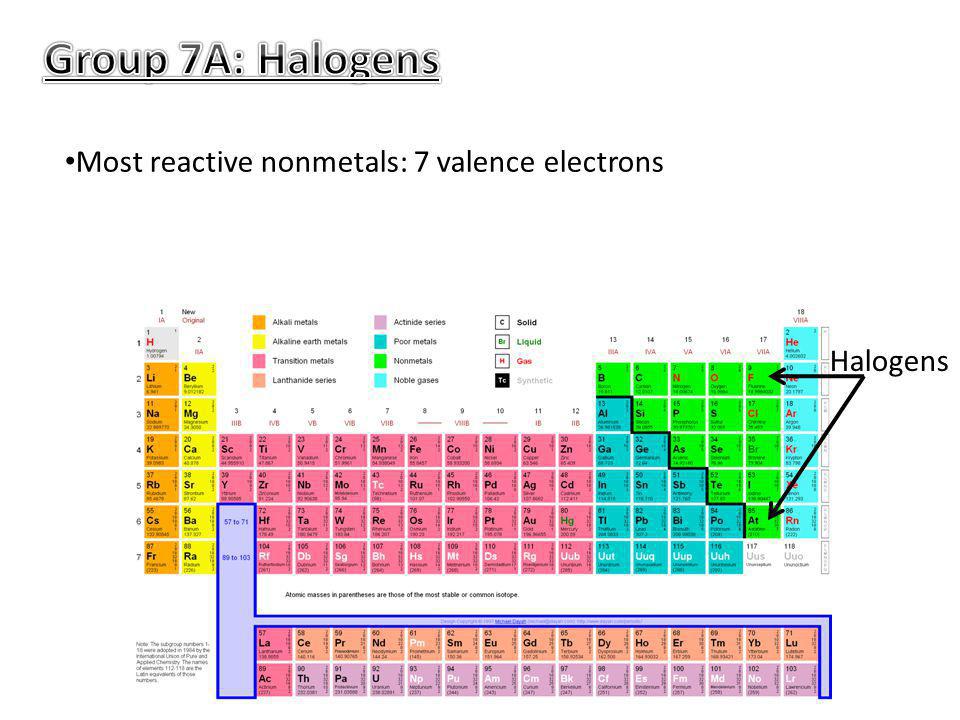
Hydrogen is a highly reactive gas and alkali metals are even more reactive. Actually the halogens are the most reactive nonmetals, but we know that as we move down the group, the electronegativity decreases. Halogens are poisonous to humans on the whole, though each one is.
 Source: brainly.com
Source: brainly.com
It is also the most reactive group of all chemical elements. Halogens are poisonous to humans on the whole, though each one is. Fluorine is the most reactive nonmetal because it is the most electronegative nonmetal in the periodic table.
 Source: unlimitededu.net
Source: unlimitededu.net
Is halogens the most reactive nonmetal? Fluorine is a halogen, which is group 17 on the periodic table, and the halogens are the most reactive nonmetals. Hydrogen is a highly reactive gas and alkali metals are even more reactive.
 Source: slideplayer.com
Source: slideplayer.com
Fluorine is the most reactive nonmetal because it is the most electronegative nonmetal in the periodic table. It is not found in nature as a free element. The most reactive element is fluorine, the first element in the halogen group.
 Source: slidetodoc.com
Source: slidetodoc.com
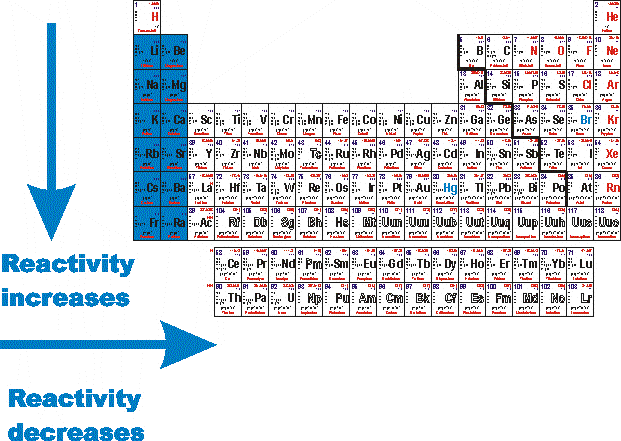
The period 2 element would be more reactive. Actually the halogens are the most reactive nonmetals, but we know that as we move down the group, the electronegativity decreases. Since the noble gases are a special group because of their lack of reactivity, the element fluorine is the most reactive nonmetal.

Due to its strong electro negativity & small size, fluorine has a strong tendency to accept electrons from other atoms or ions. Due to its strong electro negativity & small size, fluorine has a strong tendency to accept electrons from other atoms or ions. The halogen group of elements is the most reactive of the nonmetals.
 Source: willowwoodlessons.weebly.com
Source: willowwoodlessons.weebly.com
Group 8a — the noble or inert gases. The most reactive nonmetal is fluorine. Fluorine is the most reactive element in this group.
 Source: socratic.org
Source: socratic.org
Caesium is the most reactive metal in the periodic table, so much that working with this metal often ends in explosions! Which metal family is the most reactive? Halogens are among the most reactive of all elements.
 Source: breakingatom.com
Source: breakingatom.com
The most reactive nonmetal is fluorine. Fluorine is the most reactive nonmetal because it is the most electronegative nonmetal in the periodic table. So it has highest electronegativity (it has maximum tendency to attract the electrons pair)
 Source: youtube.com
Source: youtube.com
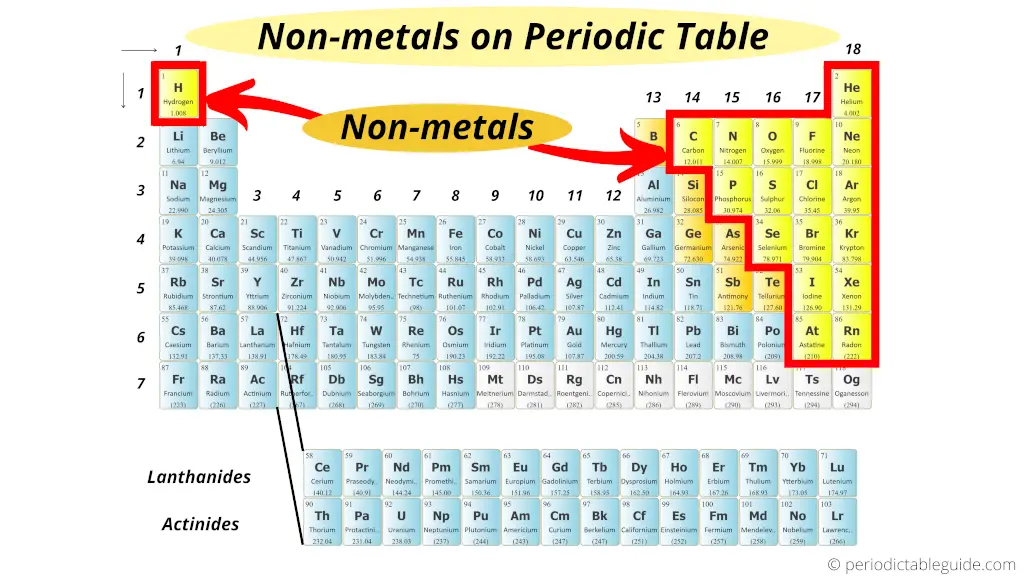
Fluorine is the only element that reacts with group viii a xenon due to its high electronegativity value. Which among the following is the most reactive metal? What is the most responsive element?
 Source: periodictableguide.com
Source: periodictableguide.com
The most reactive metal is francium, the last alkali metal (and most expensive element). The most reactive nonmetal is fluorine. Reactivity of group 1 elements hydrogen is a very reactive gas, and the alkali metals are even more reactive.
 Source: periodictableguide.com
Source: periodictableguide.com
The halogen group of elements is the most reactive of the nonmetals. Fluorine is the only element that reacts with group viii a xenon due to its high electronegativity value. Which among the following is the most reactive metal?

The period 2 element would be more reactive because the attractive force of protons is stronger when there are fewer neutrons interfering. Caesium is the most reactive metal in the periodic table, so much that working with this metal often ends in explosions! Helium (he), neon (ne), argon (ar), krypton (kr), xenon (xe), and radon (rn).
 Source: slidetodoc.com
Source: slidetodoc.com
In fact, they are the most reactive metals and, along with the elements in group 17, the most reactive of all the elements. The most reactive element is fluorine, the first element in the halogen group. What is the most responsive element?
 Source: slideplayer.com
Source: slideplayer.com
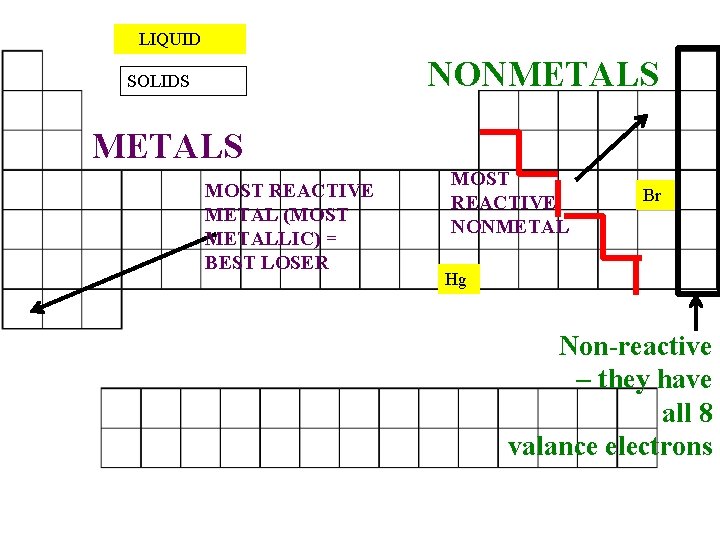
The most reactive element in period 2 is lithium (atomic number = 3) since it an alkali metal (group 1) electronic configuration = (2,1) thanks. Which block element is highly reactive? The most reactive nonmetals reside in the upper right portion of the periodic table.
 Source: slideplayer.com
Source: slideplayer.com
This is because they all have one empty space in their valence electron shells. Grendeldekt and 3 more users found this answer helpful. This is because they all have one empty space in their valence electron shells.
 Source: scienceabc.com
Source: scienceabc.com
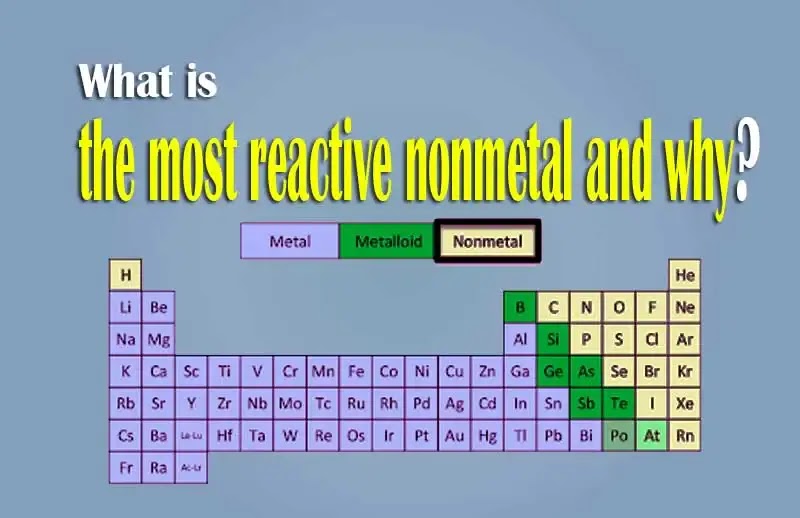
The most reactive nonmetal is fluorine. What is the most responsive element? Fluorine is the most reactive nonmetal because it has the highest value of electronegativity, reactivity of nonmetals is directly proportional to electronegativity
Also Read :





