Click here👆to get an answer to your question ️ 31. An element a (not it's real symbol) is in the family that has the most striking display of changing metallic character going down a column.
Which Element Is The Most Metallic. Learn chemistry +1 classification of elements and periodicity in properties. In the periodic table, reactivity decreases from left to right and increases from top to bottom. (boron is the first member of the group and thallium is the last.) boron. The element in period 3 with the most metallic character is.
 Of The Elements Below, ______ Is The M… | Clutch Prep From clutchprep.com
Of The Elements Below, ______ Is The M… | Clutch Prep From clutchprep.com
Related Post Of The Elements Below, ______ Is The M… | Clutch Prep :
Francium is the element with the highest metallic character. Metallic character is a periodic table trend. List of elements atomic number name symbol group period number block state at. The alkaline earth element having the largest atomic radius is found in period 1, 2, 6, 7.
Some elements which shows metallic character are listed below.
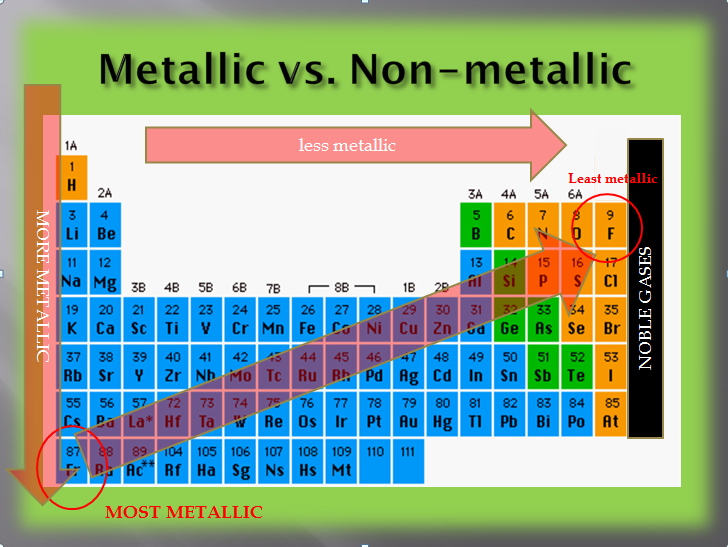
93 rows over 75% of the elements are metals, so they fill most of the periodic. At room temperature which substance is the best conductor of heat. The most metallic element is francium. (a) 1s2 2s2 2p6 3s1 (b) 1s2 2s2 2p5 (c) 1s2 2s2 2p6 3s2 (d) 1s2 2s2 2p3. Metallic character is a periodic table trend. Osmium is a chemical element with os symbol and 76 atomic number.
 Source: youtube.com
Source: youtube.com
A group of the element is the periodic table are given below. Answer the following question in relation to the above of element;. Which of the following group 2 (iia) elements has the most metallic character?
 Source: quora.com
Source: quora.com
When the electron cloud of a molecule is easily The most metallic element is francium. The most metallic element is francium.
 Source: sciencenotes.org
Source: sciencenotes.org
Asked by affaan 10/10/2018 last modified 26/11/2018. Which of the following group 2 (iia) elements has the most metallic character? Which of the following atoms will lose an electron most readily?
 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
It is not really a member of any particular group. So, thallium is most active metal among all. From na, p, ca, br:
 Source: slideplayer.com
Source: slideplayer.com
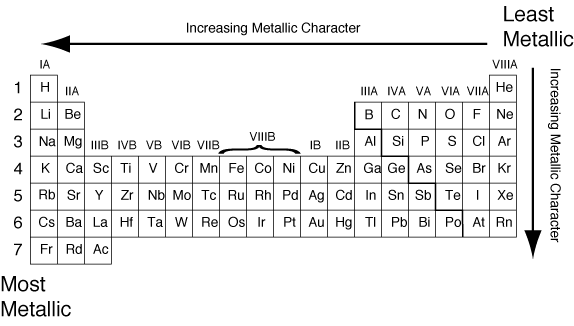
Recall that the trend for metallic character is as follows: Hydrogen is unique among the elements because _____. List of elements atomic number name symbol group period number block state at.
 Source: slideplayer.com
Source: slideplayer.com
A group of the element is the periodic table are given below. In the given elements f, p, ba, and al, which one is the most electronegative? Metallic character is a periodic table trend.
 Source: chemistrybytes.com
Source: chemistrybytes.com
A compound formed between these two elements is most likely to have the formula In the periodic table, reactivity decreases from left to right and increases from top to bottom. The elements of the third period of the periodic table are given below:
 Source: socratic.org
Source: socratic.org
Click here👆to get an answer to your question ️ 31. The natural element with the highest metallic character is cesium, which is found directly above francium on the periodic table. Recall that the trend for metallic character is as follows:
 Source: thoughtco.com
Source: thoughtco.com
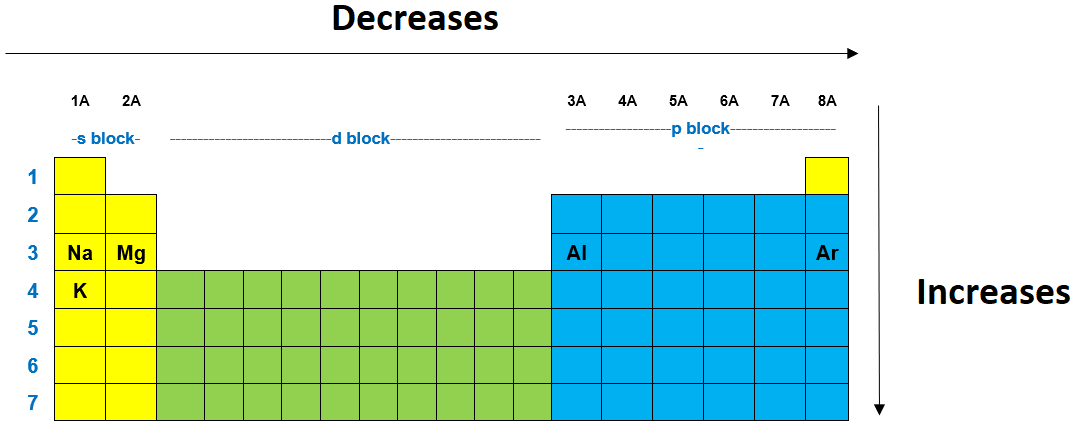
The most metallic element is francium. This is because the capacity to lose electrons from the outermost orbit of an atom (called ionisation energy) decreases. Sodium (furthest from fluorine) when a potassium atom reacts with a bromine atom, the potassium atom will.
 Source: clutchprep.com
Source: clutchprep.com
In the given elements f, p, ba, and al, which one is the most electronegative? Its electron is not at all shielded from its nucleus. List of elements atomic number name symbol group period number block state at.
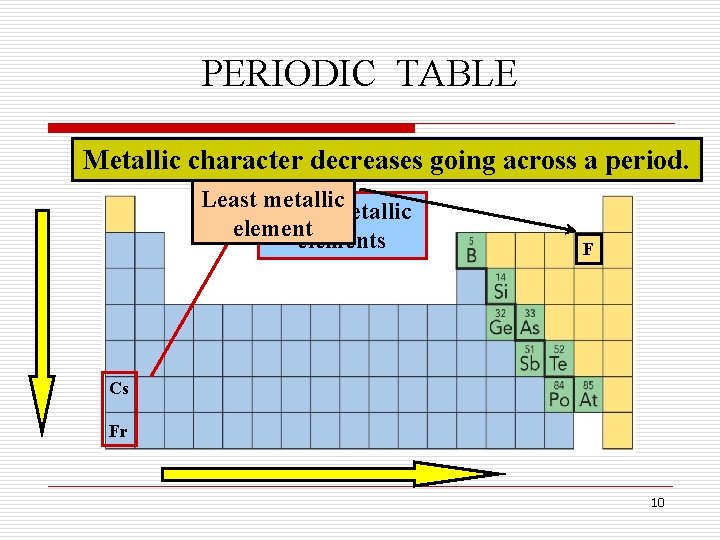 Source: slidetodoc.com
Source: slidetodoc.com
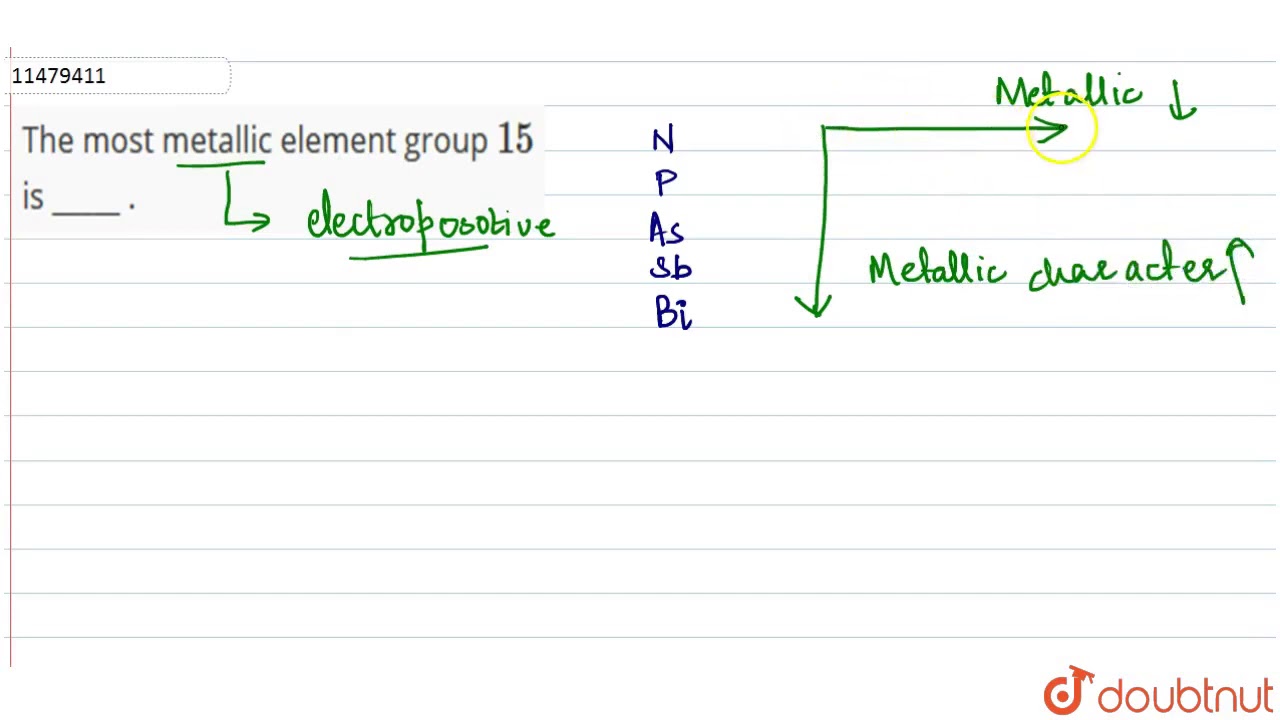
Which of the group 15 (va) elements can lose an electron most readily? The natural element with the highest metallic character is cesium, which is found directly above francium on the periodic table. The element in period 3 with the most metallic character is.
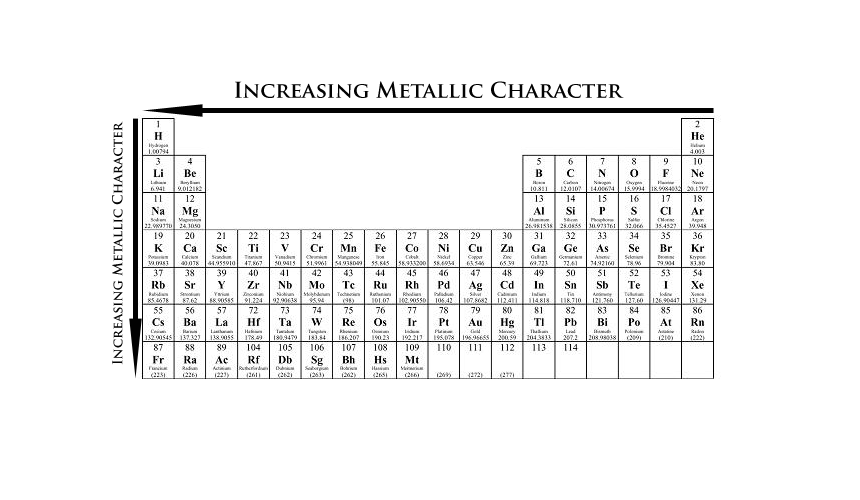 Source: 170188733453308075.weebly.com
Source: 170188733453308075.weebly.com
A compound formed between these two elements is most likely to have the formula Some elements which shows metallic character are listed below. The natural element with the highest metallic character is cesium, which is found directly above francium on the periodic table.
 Source: xaktly.com
Source: xaktly.com
It is the only element to exist at room temperature as a diatomic gas. Metallic character is a periodic table trend. It is the only element to exist at room temperature as a diatomic gas.
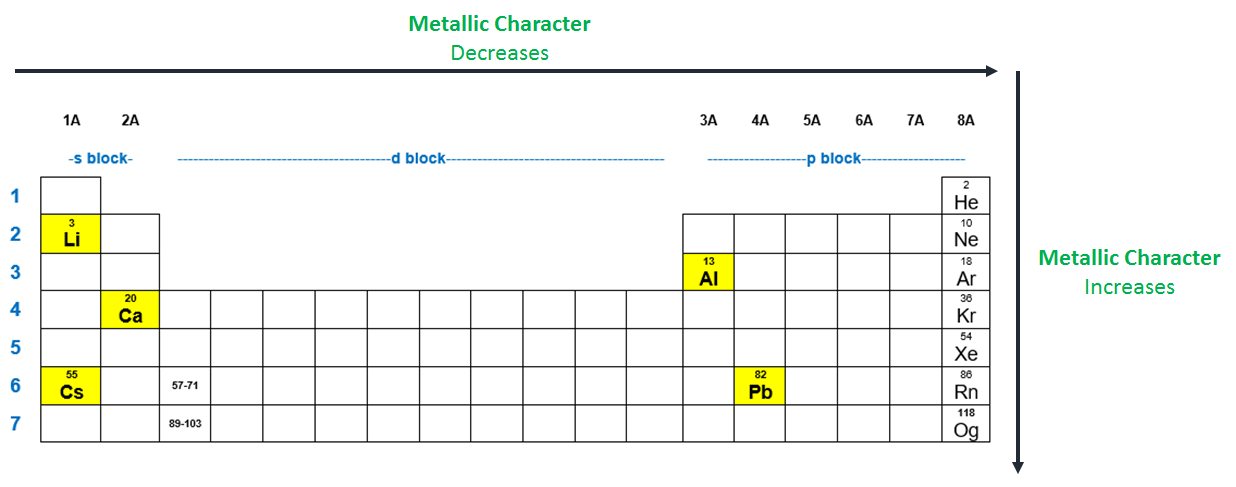 Source: clutchprep.com
Source: clutchprep.com
The most metallic element is francium. Osmium is a chemical element with os symbol and 76 atomic number. An element a (not it�s real symbol) is in the family that has the most striking display of changing metallic character going down a column.
 Source: slideplayer.com
Source: slideplayer.com
It exhibits some chemical properties similar to those of groups 1a and 7a. Hydrogen is unique among the elements because _____. In the periodic table, reactivity decreases from left to right and increases from top to bottom.
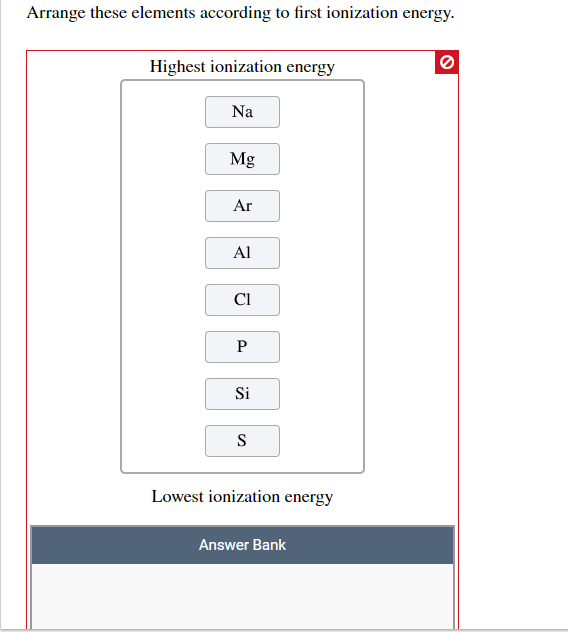 Source: chegg.com
Source: chegg.com
Which of the group 15 (va) elements can lose an electron most readily? Which element is most reactive potassium aluminum or iron? Asked by affaan 10/10/2018 last modified 26/11/2018.
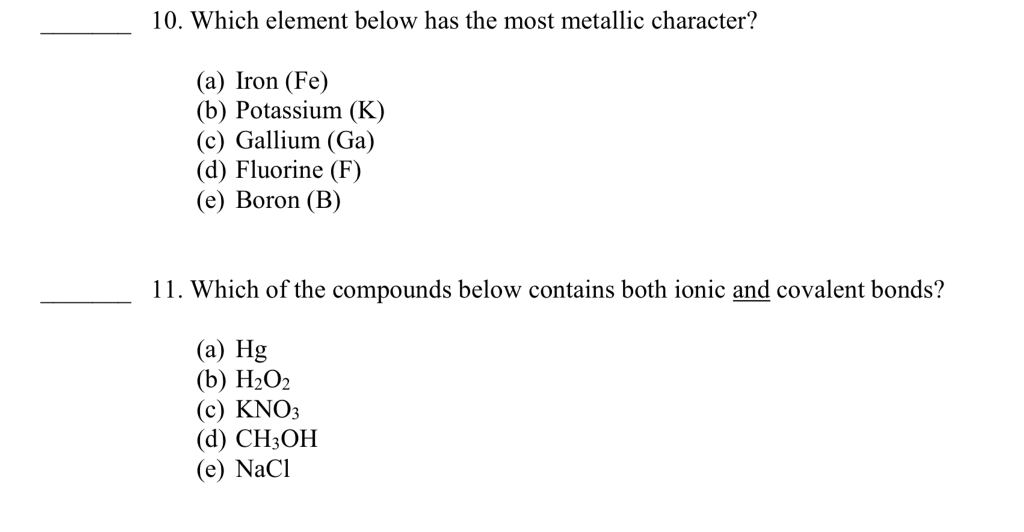 Source: chegg.com
Source: chegg.com
93 rows over 75% of the elements are metals, so they fill most of the periodic. Which of the following group 2 (iia) elements has the most metallic character? (boron is the first member of the group and thallium is the last.) boron.
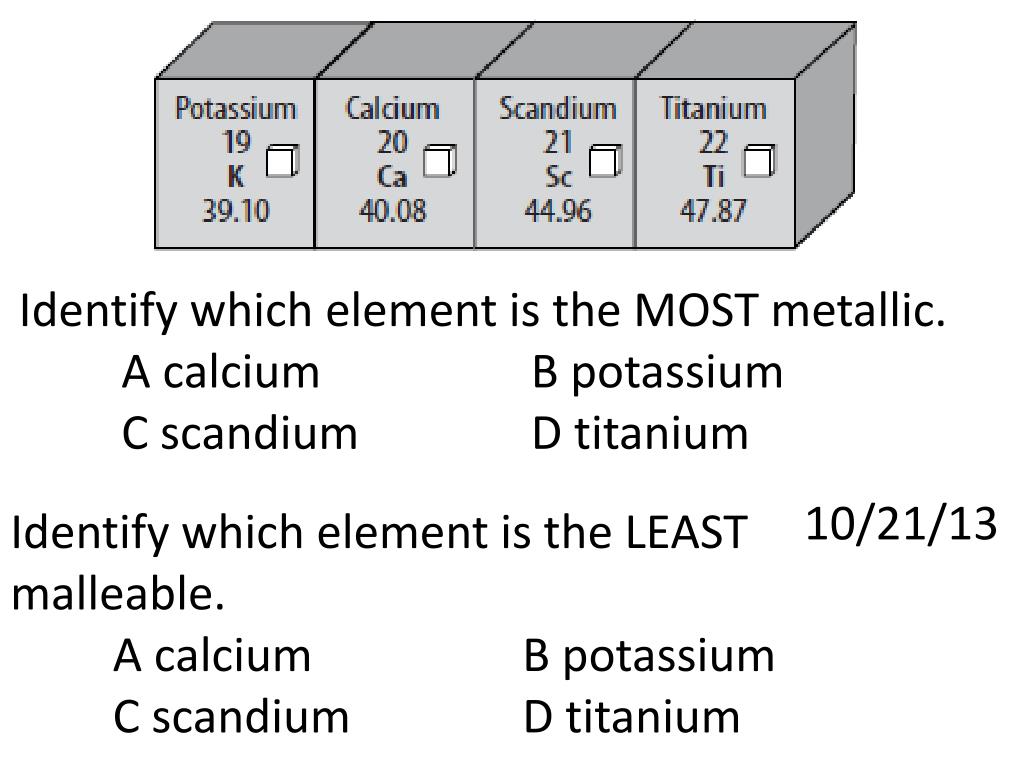 Source: slideserve.com
Source: slideserve.com
Justify your answer in each case. Some elements which shows metallic character are listed below. In the periodic table, reactivity decreases from left to right and increases from top to bottom.
 Source: clutchprep.com
Source: clutchprep.com
From na, p, ca, br: The elements with the most metallic character are on the left side of the periodic table (except hydrogen). Some elements which shows metallic character are listed below.
 Source: brainly.com
Source: brainly.com
The alkali metals in group 1 are the most active metals, and cesium is the last element in the group for which we have experimental data. Francium is the element with the highest metallic character. Its electron is not at all shielded from its nucleus.
Also Read :





