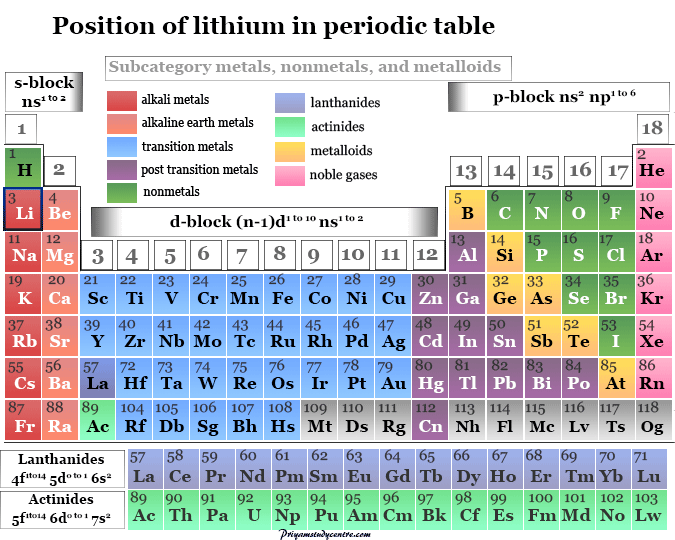
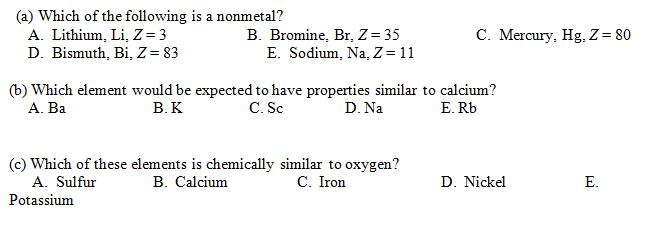
Which element is chemically similar to lithium? The alkali metals consist of the chemical elements lithium (li), sodium (na), potassium (k), rubidium (rb), caesium (cs), and francium (fr).
Which Element Is Chemically Similar To Lithium. Halogens are a group of elements: Based on these data, what is the mass of the. Li lithium and k potassium both belong to first group (alkali metals) and thus have similar chemical properties. Which element is chemically similar to lithium?
 Solved Which Element Is Chemically Similar To Lithium? A) | Chegg.com From chegg.com
Solved Which Element Is Chemically Similar To Lithium? A) | Chegg.com From chegg.com
Related Post Solved Which Element Is Chemically Similar To Lithium? A) | Chegg.com :
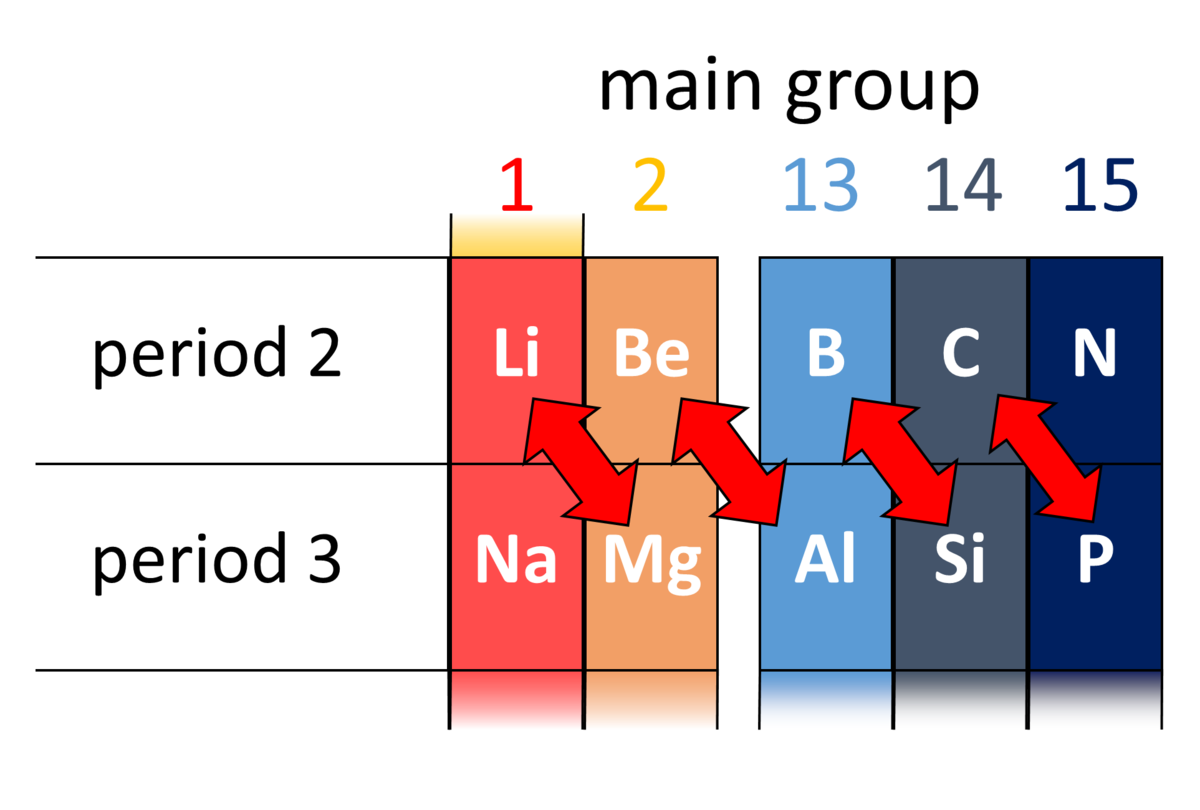
Which element is chemically similar to magnesium? These pairs (lithium (li) and magnesium (mg), beryllium (be) and aluminium (al), boron (b) and silicon (si), etc.) exhibit similar properties; Name four elements that have properties similar to hydrogen. They are gallium and indium.
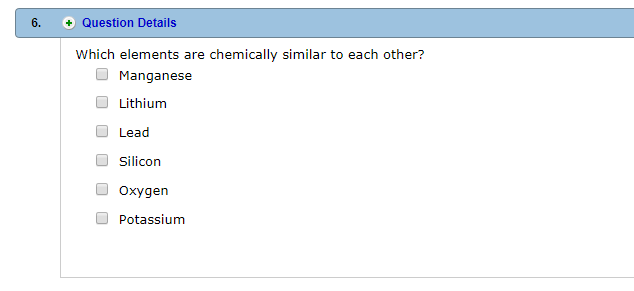
Which element is chemically similar to lithium?
The metal itself—which is soft, white, and lustrous—and several of its alloys and compounds are produced on an industrial scale. The metal itself—which is soft, white, and lustrous—and several of its alloys and compounds are produced on an industrial scale. As the elements in group 17 are considered in order of increasing atomic number, the chemical reactivity of each successive element a) remains the same b) decreases c) increases 9. S ar he la none of these elements is an s block element. 79 protons, 79 electrons, 39 neutrons d. Tl;dr this question is fundamentally flawed.
 Source: chegg.com
Source: chegg.com
The elements of this family they all have similar chemical properties. Which element is chemically similar to lithium? 38 p, 52 n b.
 Source: dreamstime.com
Source: dreamstime.com
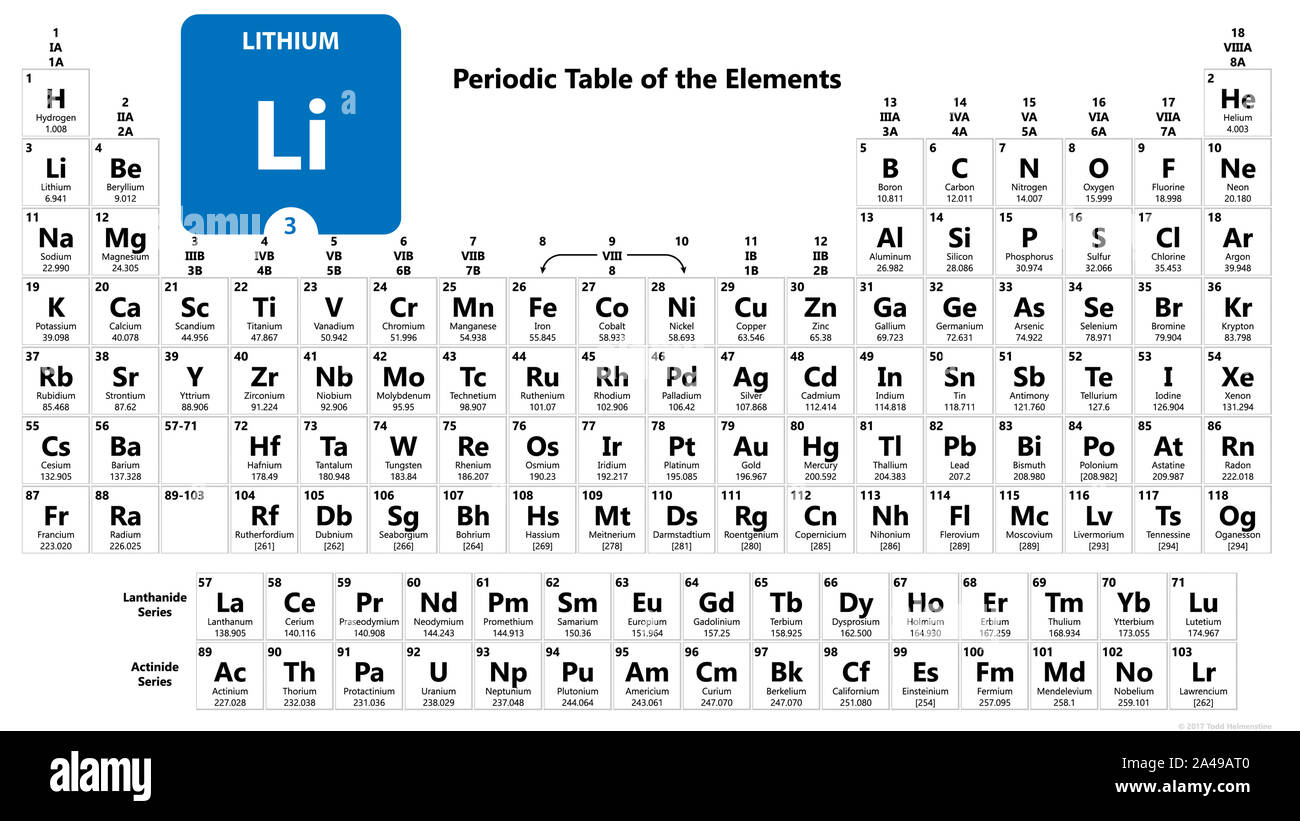
These are sodium, potassium, rubidium, cesium and francium. Which element is chemically similar to lithium? Elements in the same column (families) usually share similar properties because they have the same number of valence electrons.
 Source: alamy.com
Source: alamy.com
For example, pairs lithium (li) and magnesium (mg), beryllium (be) and aluminium (al), boron (b) and silicon (si), etc are exhibiting similar properties. Halogens are a group of elements: The alkali metals consist of the chemical elements lithium (li), sodium (na), potassium (k), rubidium (rb), caesium (cs), and francium (fr).
 Source: sciencing.com
Source: sciencing.com
The metal itself—which is soft, white, and lustrous—and several of its alloys and compounds are produced on an industrial scale. 118 protons, 118 electrons, 79 neutrons b. S ar ag as none of these elements is a d block element.
![Solved] Extension: Get The Gizmo Ready: . Select Lithium (Li) From The Select A Metal List. Bo… Chemical Families You Will Need A Periodic Table F… | Course Hero](https://www.coursehero.com/qa/attachment/16767640/ “Solved] Extension: Get The Gizmo Ready: . Select Lithium (Li) From The Select A Metal List. Bo… Chemical Families You Will Need A Periodic Table F… | Course Hero”) Source: coursehero.com
Which element is chemically similar to lithium? Which element is chemically similar to magnesium? But none of them have similar chemical properties.
 Source: britannica.com
Source: britannica.com
Check out your periodic table. The elements of this family they all have similar chemical properties. Name four elements that have properties similar to hydrogen.
 Source: priyamstudycentre.com
Source: priyamstudycentre.com
Tl;dr this question is fundamentally flawed. Which element is chemically similar to lithium? Which electron configurations represent the first two elements in group 17 (viia) of the periodic table?
 Source: chegg.com
Source: chegg.com
Which element is chemically similar to lithium? The symbol for gold is au. 38 p, 90 n c.
 Source: numerade.com
Source: numerade.com
Which atom is most like h? Their oxidation numbers including aluminum is 3. Which element has similar properties to lithium explain?
 Source: en.wikipedia.org
Source: en.wikipedia.org
What is chemically similar to oxygen? The least reactive elements are the noble gases. Which element is most chemically similar to chlorine?
 Source: priyamstudycentre.com
Source: priyamstudycentre.com
Which element is most chemically similar to chlorine? Flourine (f), chlorine (cl), bromine (br), iodine (i) and astatine (at). The alkali metals consist of the chemical elements lithium (li), sodium (na), potassium (k), rubidium (rb), caesium (cs), and francium (fr).
 Source: slideplayer.com
Source: slideplayer.com
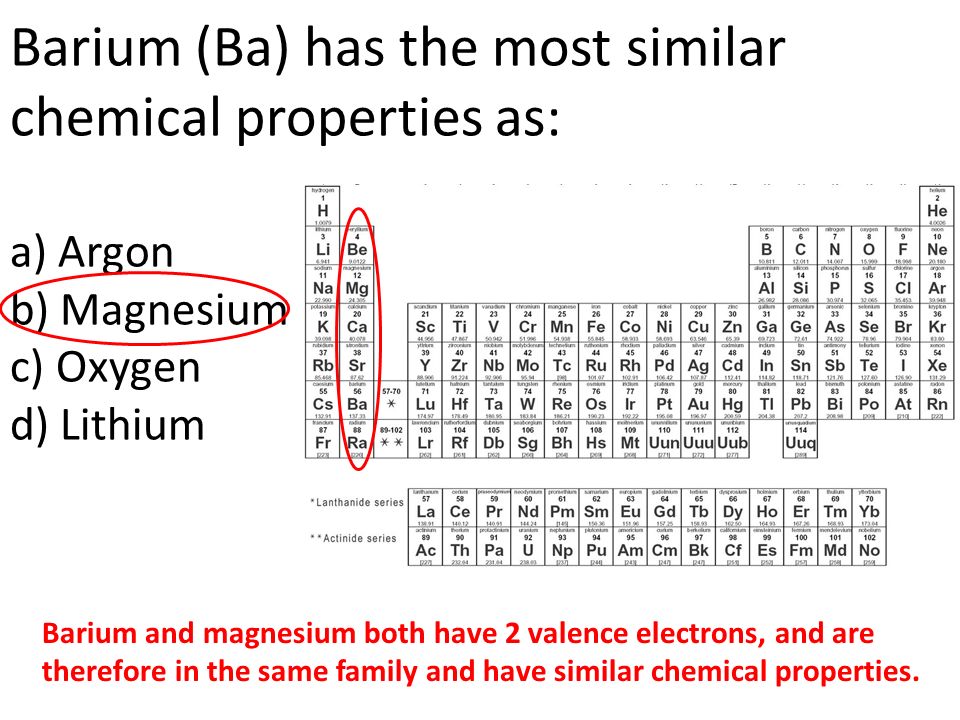
An atom with a=21 and z=10 is an isotope of an atom with a=20 and z=10. Halogens are a group of elements: Strontium and barium have similiar chemical properties.

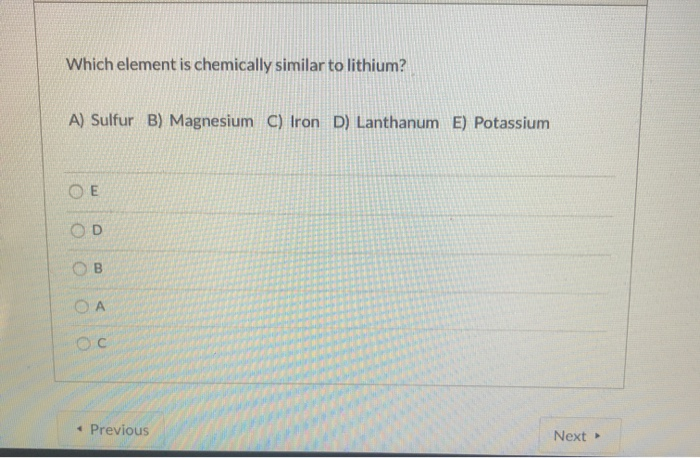
All the elements in group two of the periodic table have the same or similar properties. A) sulfur b) magnesium c) iron d) lanthanum e) potassium Strontium and barium have similiar chemical properties.

Which electron configurations represent the first two elements in group 17 (viia) of the periodic table? How many protons (p) and neutrons (n) are in an atom of sr? They’re both gases at room temperature, while arsenic and sulfur are solids.
 Source: chegg.com
Source: chegg.com
How many protons (p) and neutrons (n) are in an atom of sr? The least reactive elements are the noble gases. Which element is chemically similar to lithium?
 Source: brainly.com
Source: brainly.com
Which element has similar properties to lithium explain? 38 p, 90 n c. 38 p, 52 n b.
 Source: coursehero.com
Source: coursehero.com
The metal itself—which is soft, white, and lustrous—and several of its alloys and compounds are produced on an industrial scale. Sodium,potassium , cesium are similar with lithium and all are mono valence. One isotope has an abundance of 57.30% and an isotopic mass of 120.904 amu.
 Source: coursehero.com
Source: coursehero.com
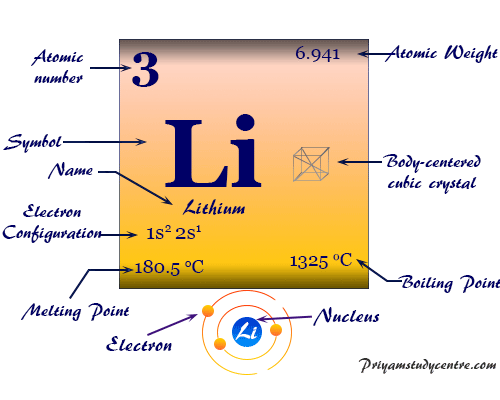
Which element has similar properties to lithium? A compound is a pure substance formed by chemically combining two or more atoms together. Why is lithium very reactive?

Elements with the same number of valence electrons will have similar properties. Lithium and potassium have properties similar to those of the element sodium because they are in the same family, or group, on the periodic table. These pairs (lithium (li) and magnesium (mg), beryllium (be) and aluminium (al), boron (b) and silicon (si), etc.) exhibit similar properties;
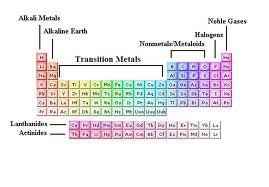 Source: quizlet.com
Source: quizlet.com
Which element is an s block element? These are sodium, potassium, rubidium, cesium and francium. The alkali metals consist of the chemical elements lithium (li), sodium (na), potassium (k), rubidium (rb), caesium (cs), and francium (fr).
Also Read :





