Which element has the smallest atomic radius? An atom of which of the following elements has the smallest atomic radius?
Which Element Has The Smallest Radius. Which element in group 2a has the lowest ionic radius? Therefore sodium (na) has the largest atomic radius. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top. Which one of the following atoms has the largest radius?

Related Post Atomic Radius Trend | Periodic Table | Chemtalk :
An old problem, and one that which the periodic table, which should be routinely available in all chemistry and fyziks examinations (and which should be in front of you now!), directly addresses. 119 rows we have shown the atomic radius of the elements for which reliable. What does atomic radius depend on? A) i b) co c) ba d) sr e) ca.
Strontium has a larger atomic radius because strontium has more shells of electrons than magnesium does.
A reasonable explanation for this fact involves. The periodic table is divided into. Which element has the smallest radius? Atomic size gradually decreases from left to. What elements have the smallest atomic radius? Helium has the smallest atomic radius.
 Source: clutchprep.com
Source: clutchprep.com
What does atomic radius depend on? Which element has the smallest radius? No of l u = 7 1, l a = 5 7 )?
 Source: slideplayer.com
Source: slideplayer.com
0 6 a o, which of the following given values will be closest to the radius of l u 3 + (at. Pairing of two electrons in one 3p orbital in sulfur atoms. 119 rows elements atomic radius of elements (pm) 1:
 Source: sciencenotes.org
Source: sciencenotes.org
As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. So, all these elements belong to the 4th group and kr has the smallest atomic radii as it lies across the period. A property that measures the ability of an atom to attract electrons in a chemical bond is.
 Source: brainly.com
Source: brainly.com
Which one of the following atoms has the largest radius? This is done by beaming a signal of a certain frequency through isolated cesium atoms. Oct 11, 2017 · the element that has the smallest atomic radius is fluorine.
 Source: blog.prepscholar.com
Source: blog.prepscholar.com
As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. What causes the atomic radius of the elements to decrease across period 3 from left to right? A) na b) cl c) p d) br e) k.
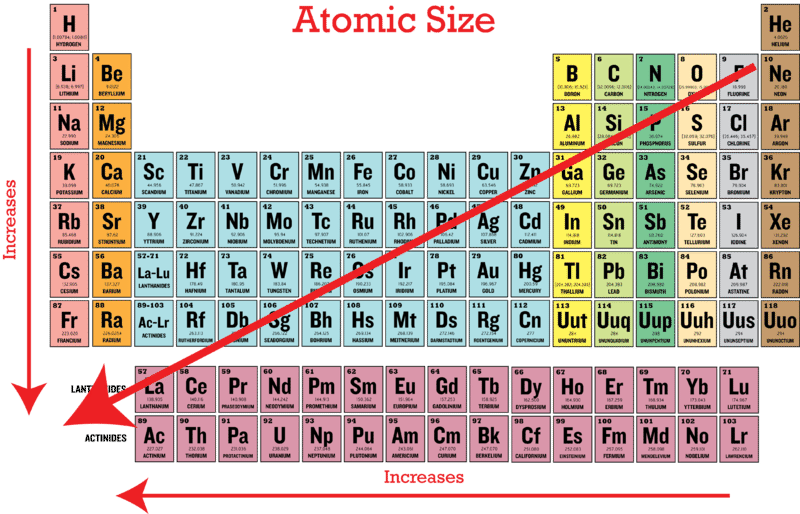
A) i b) co c) ba d) sr e) ca. It not only tells you names of elements a, b, c, d but also their groups and periods. Which of the lists of increasing ionic radii is not correct?
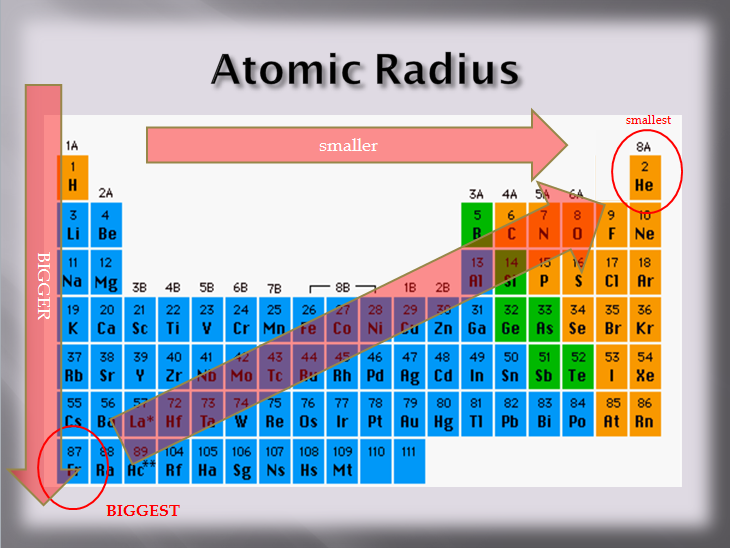 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
An atom of which of the following elements has the largest atomic radius? A) i b) co c) ba d) sr e) ca. Atomic size gradually decreases from left to.
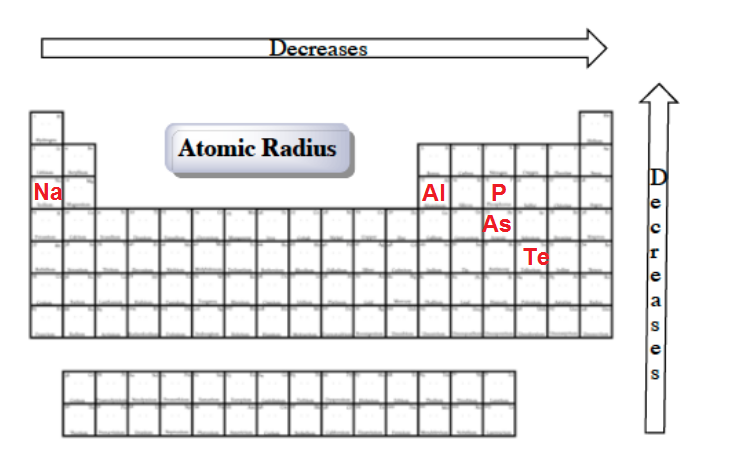 Source: clutchprep.com
Source: clutchprep.com
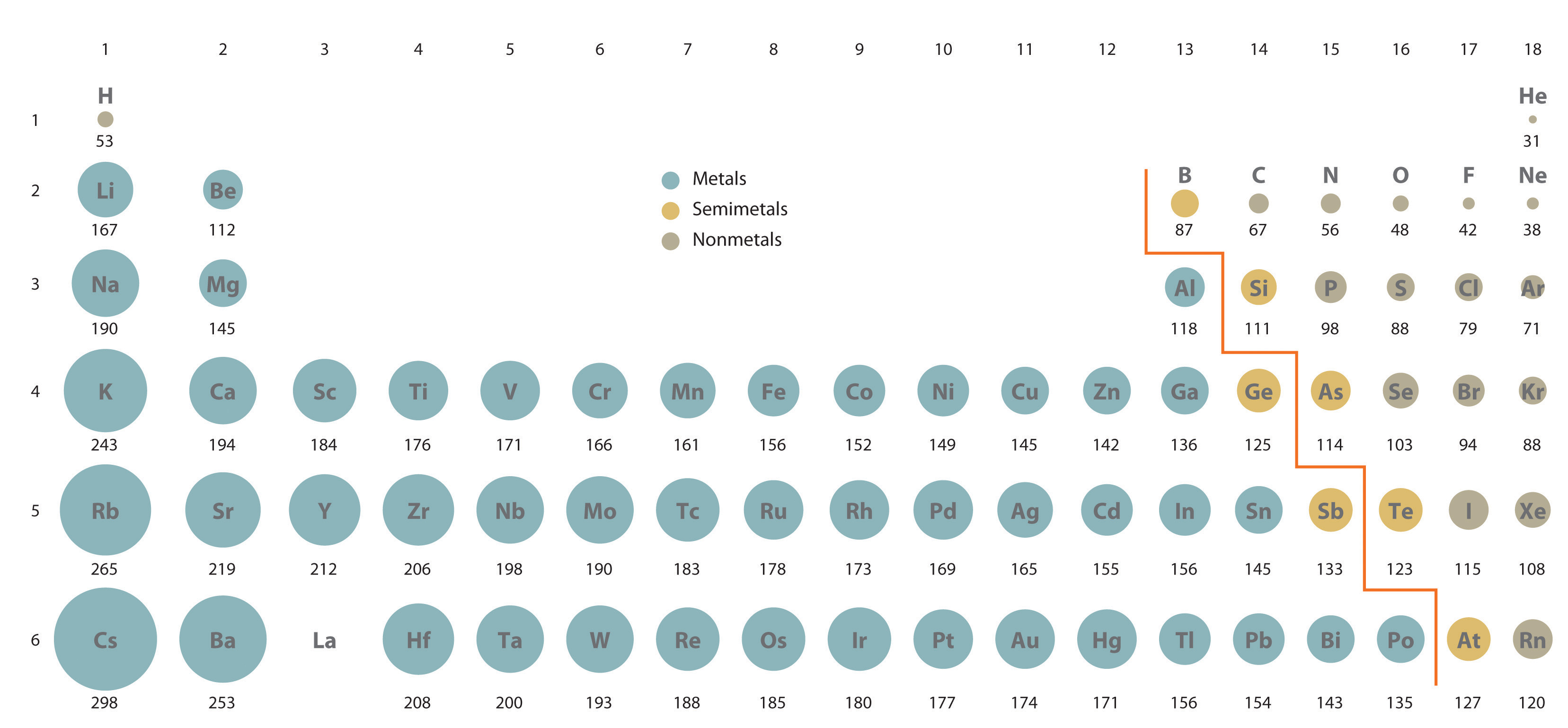
Periodic table of elements with covalent radius trends. Lithiumso, for period 2, the group1 alkali metal (lithium, lowest z) has the largest atomic radius and the group 7/17 halogens & group 0/18 noble gas (fluorine & neon, highest z’s) have the smallest atomic radii (there is some uncertainty in the noble gas radii). Which element has the smallest atomic radius?

Which one of the following elements has the largest atomic radius? Lithiumso, for period 2, the group1 alkali metal (lithium, lowest z) has the largest atomic radius and the group 7/17 halogens & group 0/18 noble gas (fluorine & neon, highest z’s) have the smallest atomic radii (there is some uncertainty in the noble gas radii). In the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column.
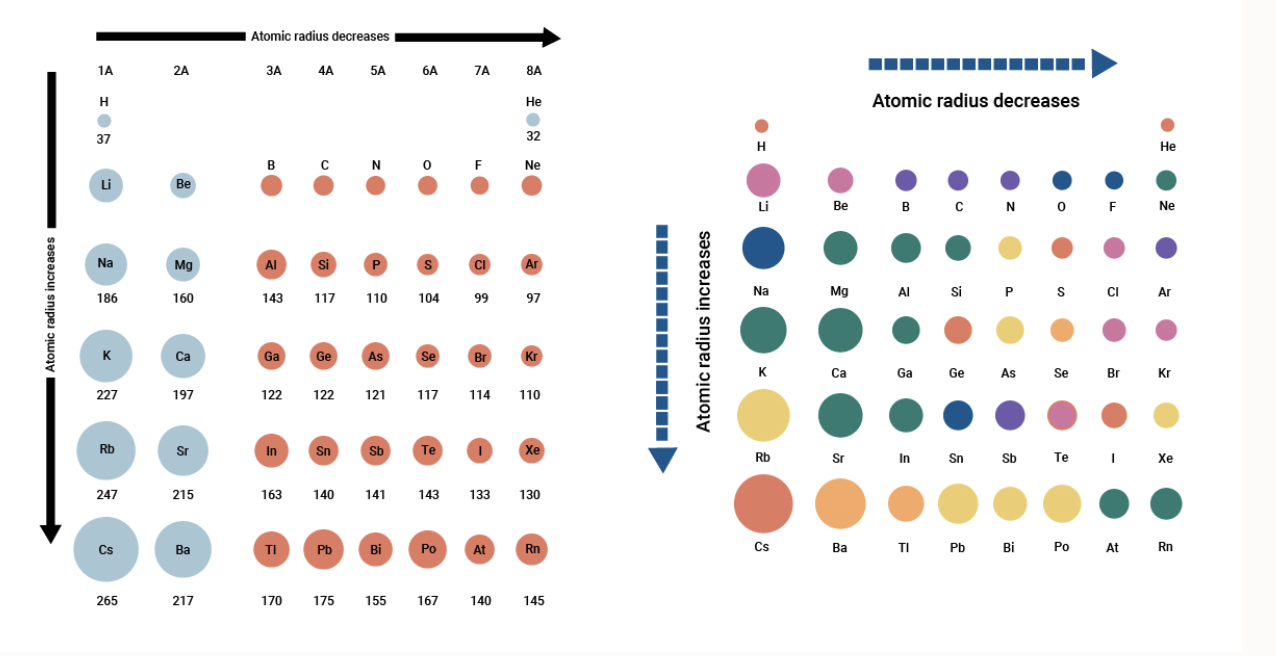 Source: breakingatom.com
Source: breakingatom.com
An atom of which of the following elements has the largest atomic radius? Periodic table of elements with covalent radius trends. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top.
 Source: angelo.edu
Source: angelo.edu
In context of the lanthanoids , which of the following statements is not correct ? Which element has the smallest atomic radius? Strontium has a larger atomic radius because strontium has more shells of electrons than magnesium does.
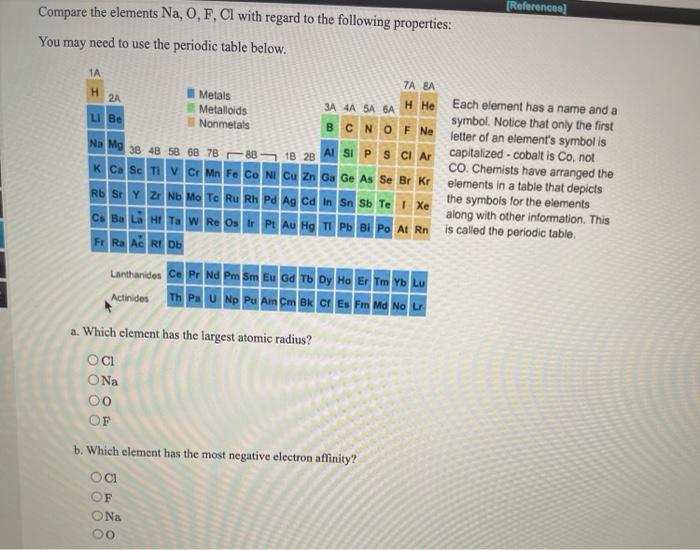
Which element has the smallest radius? S2− < cl− < k+. For facts, physical properties, chemical properties, structure and atomic properties of the specific element, click.
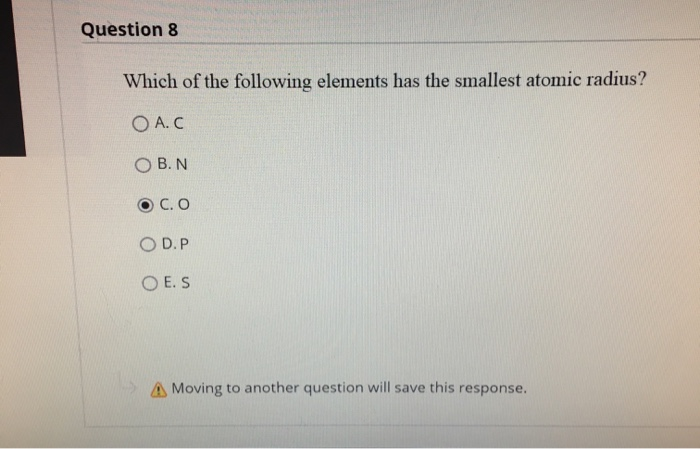 Source: chegg.com
Source: chegg.com
What does atomic radius depend on? A) i b) co c) ba d) sr e) ca. This is done by beaming a signal of a certain frequency through isolated cesium atoms.

What does atomic radius depend on? This is done by beaming a signal of a certain frequency through isolated cesium atoms. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top.
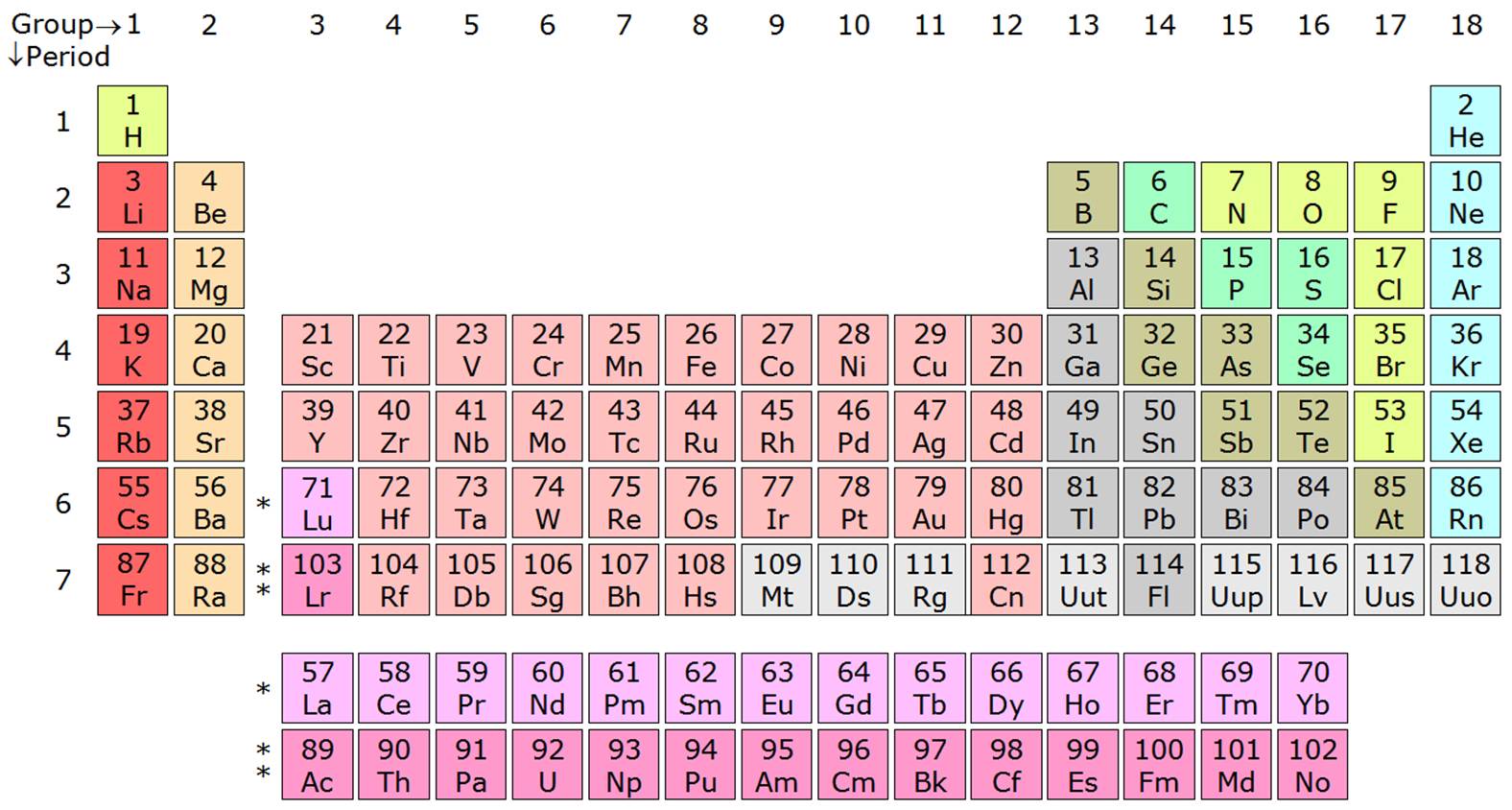 Source: socratic.org
Source: socratic.org
Helium has the smallest atomic radius. For facts, physical properties, chemical properties, structure and atomic properties of the specific element, click. Which element has the smallest atomic radius?

The periodic table is provided! Which of the lists of increasing ionic radii is not correct? A) i b) co c) ba d) sr e) ca.
 Source: chegg.com
Source: chegg.com
Helium has the smallest atomic radius. Atomic size gradually decreases from left to. An atom of which of the following elements has the largest atomic radius?
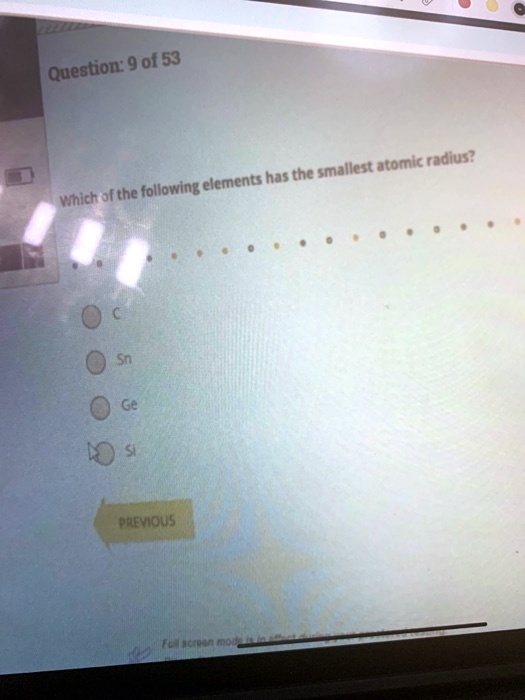 Source: numerade.com
Source: numerade.com
Lithiumso, for period 2, the group1 alkali metal (lithium, lowest z) has the largest atomic radius and the group 7/17 halogens & group 0/18 noble gas (fluorine & neon, highest z’s) have the smallest atomic radii (there is some uncertainty in the noble gas radii). What does atomic radius depend on? Strontium has a larger atomic radius because strontium has more shells of electrons than magnesium does.
 Source: socratic.org
Source: socratic.org
A reasonable explanation for this fact involves. Helium has the smallest atomic radius. Pairing of two electrons in one 3p orbital in sulfur atoms.
 Source: youtube.com
Source: youtube.com
The first ionization energy of sulfur is less than that of a phosphorus. Which elements have the smallest radius? Therefore sodium (na) has the largest atomic radius.
Also Read :





