The third ionization energy is the energy it takes to remove an electron from a 2+ ion. The first ionisation energy of magnesium is less than that of aluminium.
Which Element Has The Smallest First Ionization Energy. For chemistry students and teachers: The first column is 6 entries, headed 1 and 1 a. What kind of element has low electronegativity? Which has lowest first ionization energy?
 7.4: Ionization Energy - Chemistry Libretexts From chem.libretexts.org
7.4: Ionization Energy - Chemistry Libretexts From chem.libretexts.org
Related Post 7.4: Ionization Energy - Chemistry Libretexts :
(c) chlorine would have the largest first ionization energy. Which of the following elements has the greatest first ionization energy? For chemistry students and teachers: First ionization energy is the energy required to separate one valence electron from an atom in gas phase.
This value uses the pauling scale to measure electronegativity.
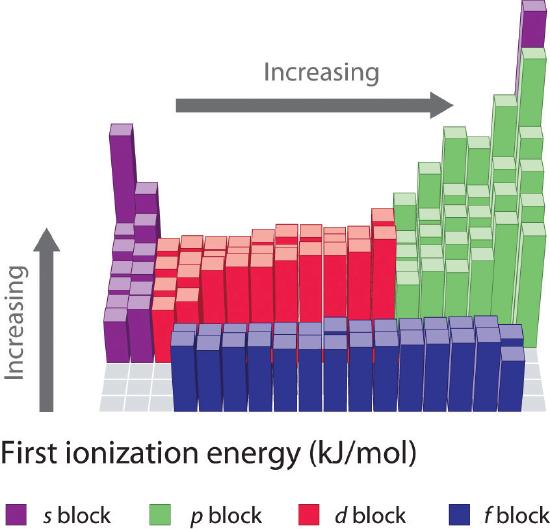
Why does group 1 have the lowest ionization energy? As a result, li has a significantly smaller first ionization energy than helium. Which element has the highest first ionization energy? The ionization energy decreases from top to bottom in groups, and increases from left to right across a period. Cesium has atomic number 55 and is in the fifth row of the periodic table. Group 1 of the periodic table features metals whose valence electron singly occupies the outermost shell, the valence shell of the given atoms.
 Source: clutchprep.com
Source: clutchprep.com
(that means that the atom has already lost two electrons, you are now removing the third.) and 2nd ionization energy is higher than 1st ionization energy, 3rd is higher than 2nd, and so forth. Since, it has more number of protons. As you move across a period, first ionization energy increases.
 Source: angelo.edu
Source: angelo.edu
Since the nuclear charge is necessarily diminished with respect to the valence shell, the alkali metals display the lowest ionization energies, and these energies (reasonably) decrease down the group. Ionization energy is the energy required to remove an electron from a gaseous atom or ion. Cesiumfrom this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon).

Cesiumfrom this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon). Now in the case of na and mg ,na has lower ionization energy and mg has higher. What kind of element has low electronegativity?
 Source: socratic.org
Source: socratic.org
Which of the following elements has the greatest first ionization energy? The lower the first ionization energy. The first chemical element is cesium and the last one is helium.
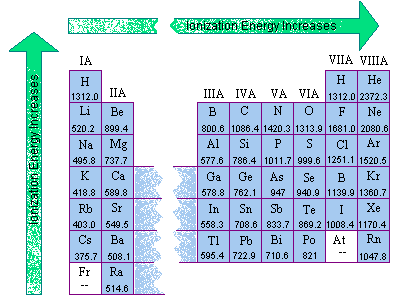 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
The alkali metals have the lowest first ionisation energies in their respective periods of the periodic table because of their low effective nuclear charge and the ability to attain a noble gas configuration by losing just one electron. Group 1 of the periodic table features metals whose valence electron singly occupies the outermost shell, the valence shell of the given atoms. (that means that the atom has already lost two electrons, you are now removing the third.) and 2nd ionization energy is higher than 1st ionization energy, 3rd is higher than 2nd, and so forth.
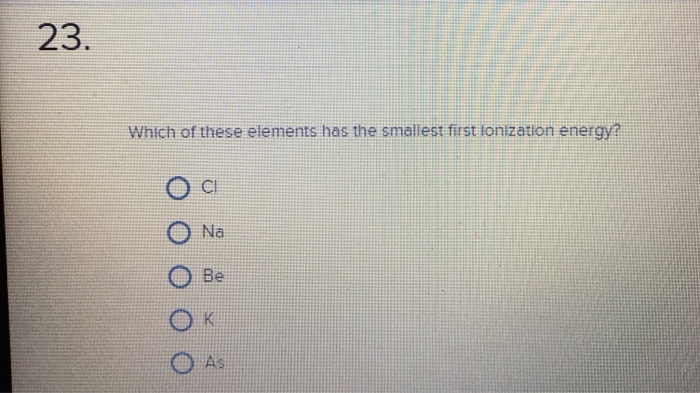 Source: chegg.com
Source: chegg.com
The first chemical element is cesium and the last one is helium. Furthermore, which group of elements has lowest ionization energy? The element with the lowest ionization energy is cesium (cs).
 Source: angelo.edu
Source: angelo.edu
As a result, li has a significantly smaller first ionization energy than helium. Which element has the smallest ionization energy? As indicated in the image attached, the lowest ionization energy�s are those of the alkali earth metals of group ia of the periodic table of the elements.
 Source: chem.libretexts.org
Source: chem.libretexts.org
The lower the first ionization energy. Furthermore, which group of elements has lowest ionization energy? The element with the lowest ionization energy is cesium (cs).
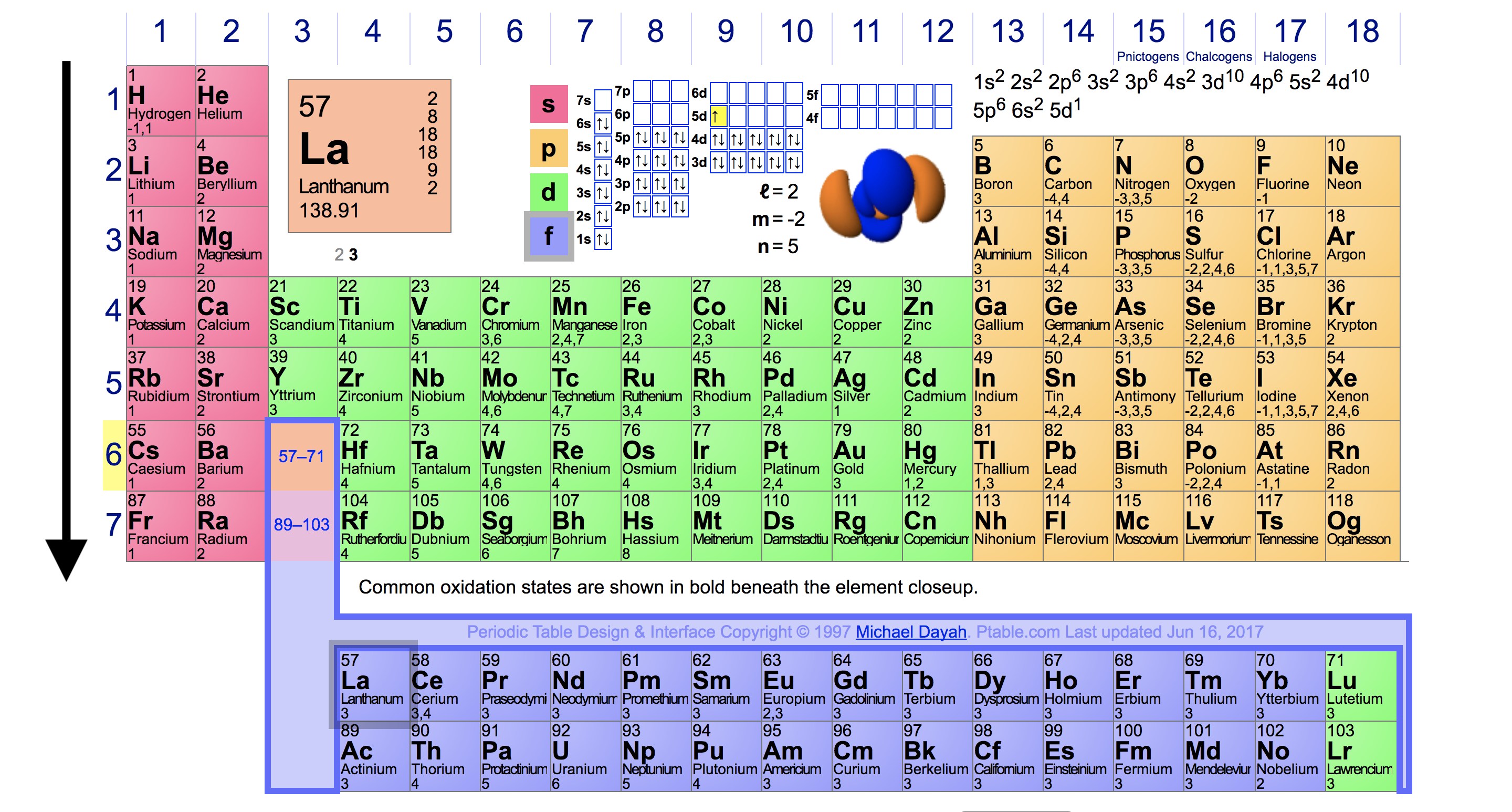 Source: socratic.org
Source: socratic.org
The first or initial ionization energy or ei of an atom or molecule is the energy required to remove one mole of electrons from one mole of isolated gaseous atoms or ions. The first chemical element is cesium and the last one is helium. The alkali metals have the lowest first ionisation energies in their respective periods of the periodic table because of their low effective nuclear charge and the ability to attain a noble gas configuration by losing just one electron.
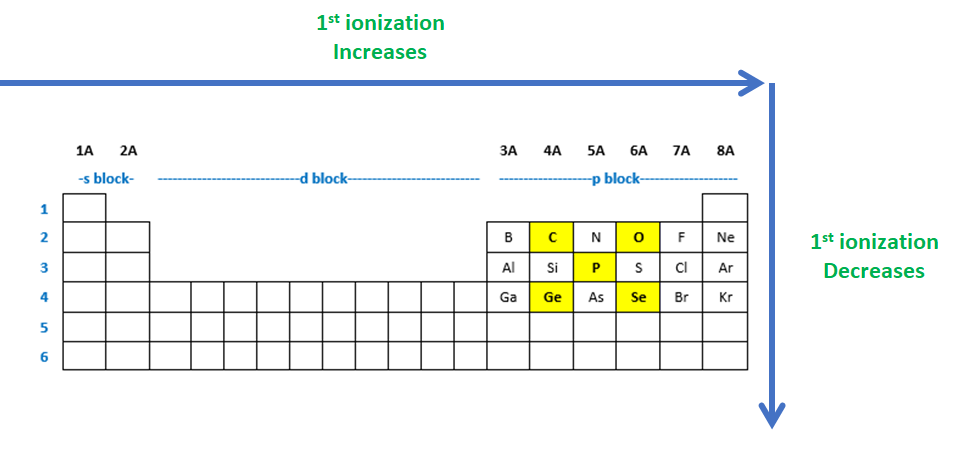 Source: clutchprep.com
Source: clutchprep.com
Since, it has more number of protons. The first chemical element is cesium and the last one is helium. Which has lower ionization energy na or k?

Ionization energy is the energy required to remove an electron from a gaseous atom or ion. An atom of which of the following elements has the smallest first ionization energy? The element with the lowest ionization energy is cesium (cs).
 Source: quizlet.com
Source: quizlet.com
(c) chlorine would have the largest first ionization energy. Which of the following elements has the greatest first ionization energy? What element has the smallest first ionization energy?

The first ionization energy of sulfur is less than that of a phosphorus. Which of these elements has the smallest first ionization energy? The ionization energy decreases from top to bottom in groups, and increases from left to right across a period.
 Source: examples.yourdictionary.com
Source: examples.yourdictionary.com
Which element has the lowest first ionization energy? Cesium has atomic number 55 and is in the fifth row of the periodic table. Wouldn�t al have the highest third ionisation energy?

Which element has the lowest ionization energy? The unity for ionization energy is ev. A reasonable explanation for this fact involves pairing of two electrons in one 3p orbital in sulfur atoms
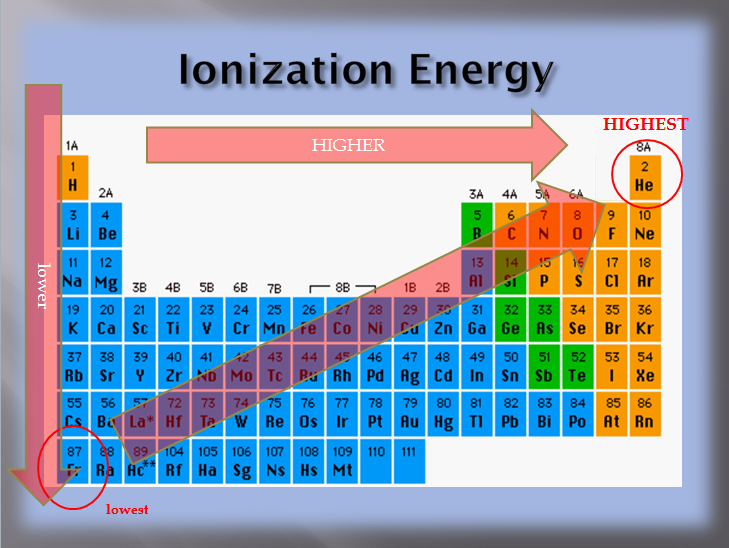 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
Which of these elements will display an unusually large jump in ionization energy values between i3 and i4, its third and fourth ionization energies? The first ionization energy of sulfur is less than that of a phosphorus. Which of the following elements has the greatest first ionization energy?
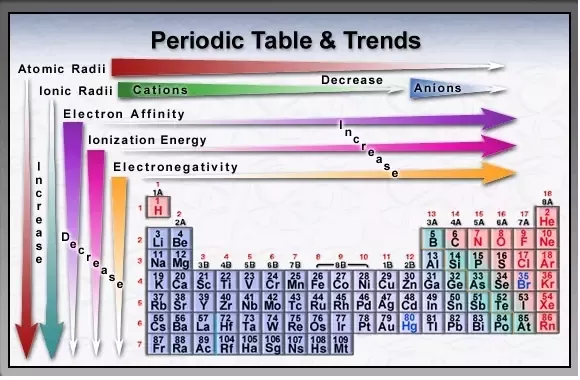 Source: quora.com
Source: quora.com
104 rows the element which has the highest ionization energy is helium with 24.58741 ev. Wouldn�t al have the highest third ionisation energy? The element with the lowest ionization energy is cesium (cs).
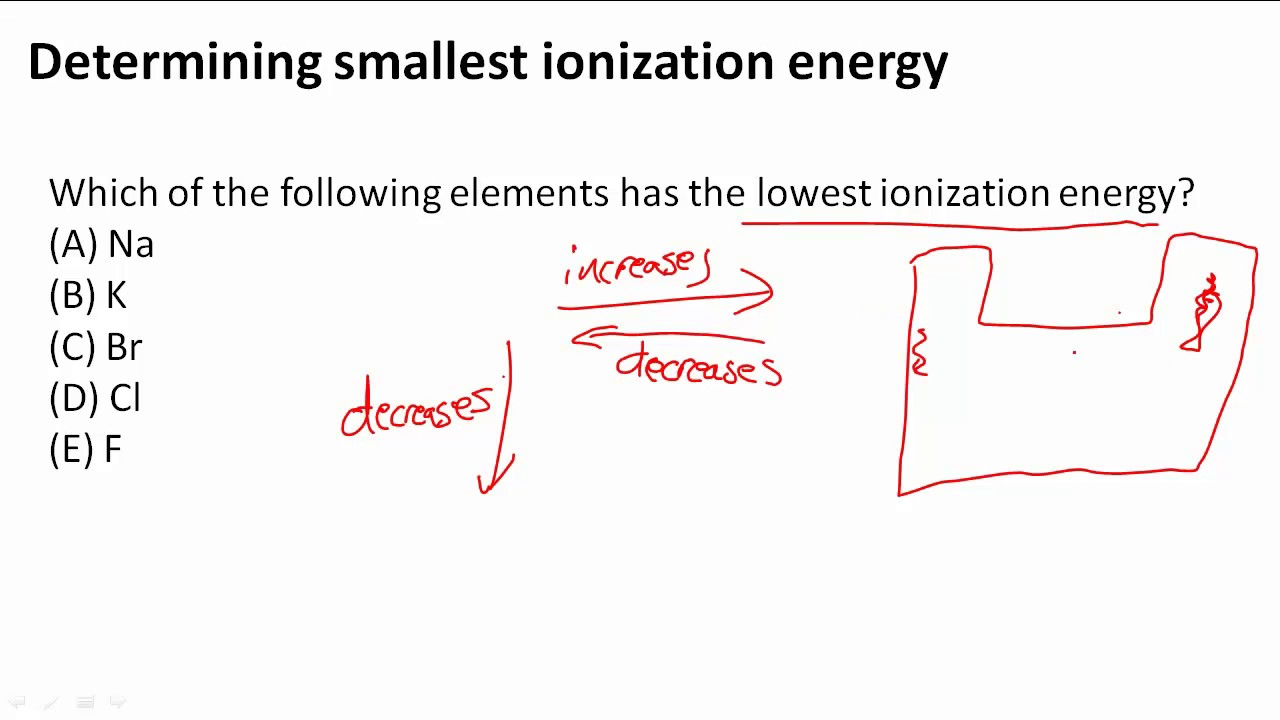 Source: youtube.com
Source: youtube.com
These include li, na and k. Besides, which element has the smallest first ionization energy? The element which has the smallest third ionisation energy:
 Source: numerade.com
Source: numerade.com
The first column is 6 entries, headed 1 and 1 a. First ionization energy is the energy required to separate one valence electron from an atom in gas phase. Besides, which element has the smallest first ionization energy?
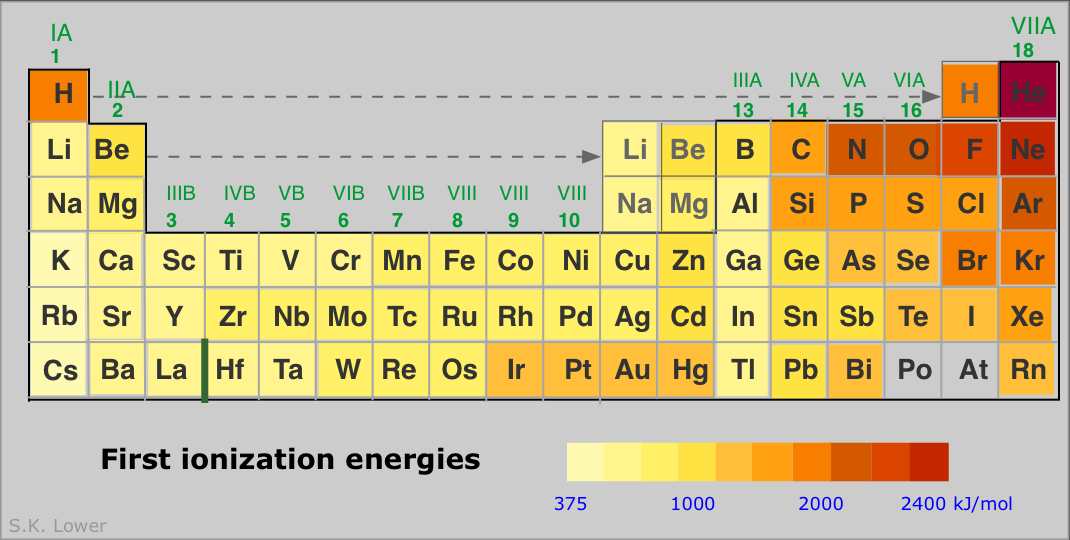 Source: chem.libretexts.org
Source: chem.libretexts.org
Cesium has atomic number 55 and is in the fifth row of the periodic table. Angelo state university › kboudrea. Since the nuclear charge is necessarily diminished with respect to the valence shell, the alkali metals display the lowest ionization energies, and these energies (reasonably) decrease down the group.
Also Read :