If we look at the periodic table group 17 and group 18 have the least or lowest metallic character. Cesium metallic character increases form right to left across a period on the periodic table, and from top to bottom down a group.
Which Element Has The Least Metallic Character. As you move down the column, the melting and boiling points _____ and the density _____. Which element has the least metallic characteristics? Thus, the elements helium, neon, fluorine,. Thus, the elements helium, neon, fluorine,.
 Chapter 9 Electrons In Atoms And The Periodic Table - Ppt Video Online Download From slideplayer.com
Chapter 9 Electrons In Atoms And The Periodic Table - Ppt Video Online Download From slideplayer.com
Related Post Chapter 9 Electrons In Atoms And The Periodic Table - Ppt Video Online Download :
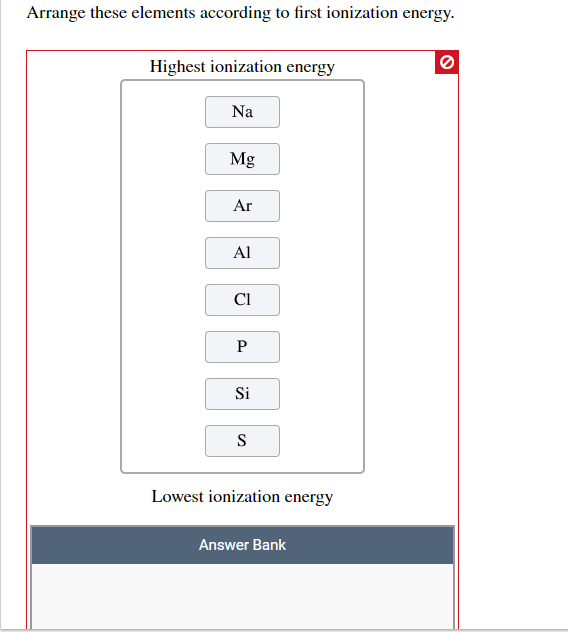
Of solid and liquid elements at normal temperature and pressure, the least metallic are those at upper right of the periodic table: Which is the correct formula for the compound formed from magnesium and sulfur? The electrons of the outermost shell experience less nuclear attraction and so can lose electrons easily thus showing increased metallic character. Arrange the elements silicon, sodium, fluorine, and cesium, in order of decreasing metallic character.
As an example, the metallic character of beryillium (4), would not be as great as the metallic character of barium (56).
It has the lowest electronegativity which means it easier loses electrons. The right and uppermost elements on the periodic table. Which element has the least metallic character. Which element has the least metallic characteristics? Which element has the least metallic character? Use the periodic table to predict which element has the least metallic character.
 Source: study.com
Source: study.com
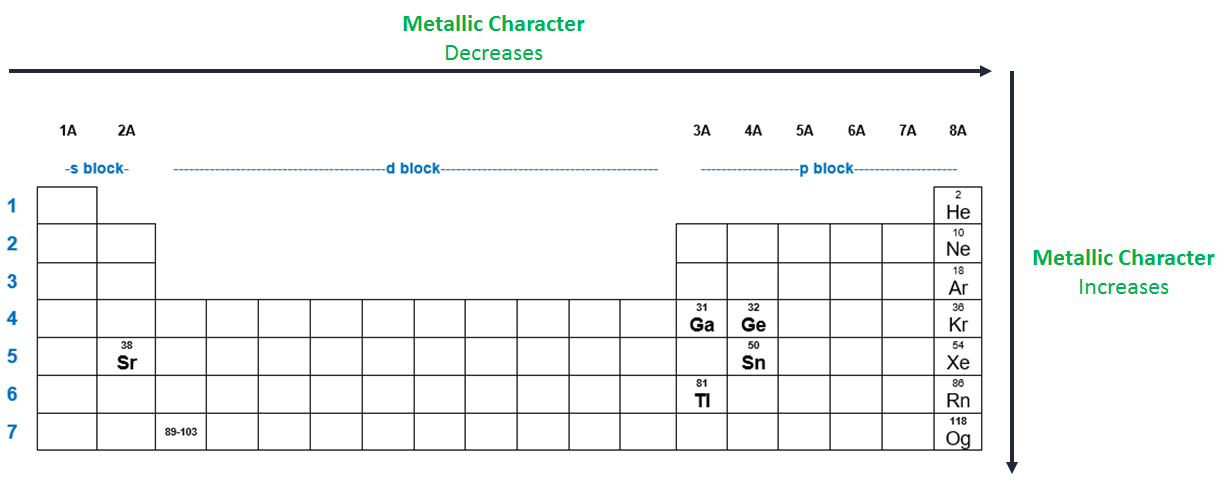
Metallic character is a periodic table trend. Thus b e has least metallic character. The alkali metals in group 1 are the most active metals, and cesium is the last element in the group for which we have experimental data.
 Source: clutchprep.com
Source: clutchprep.com
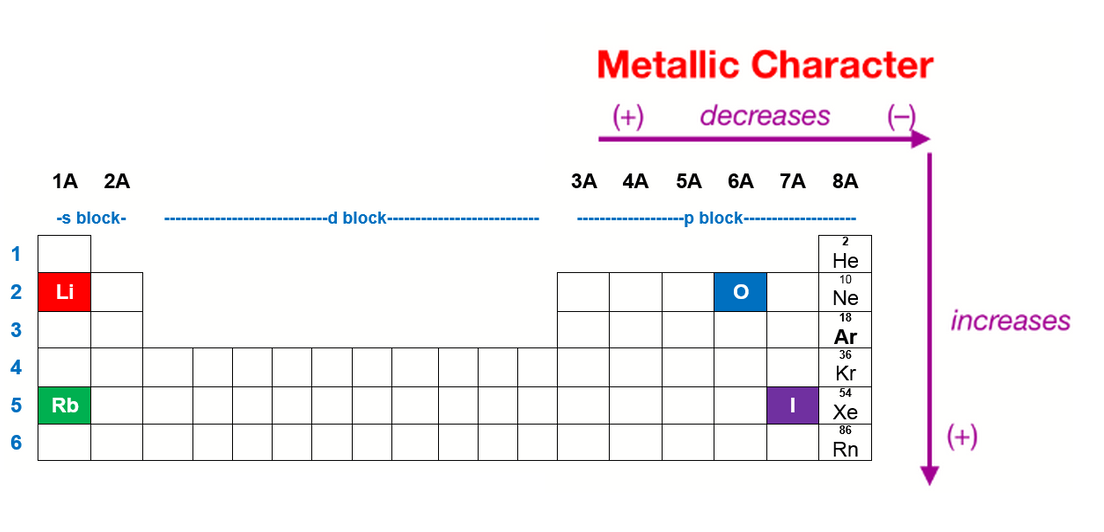
Group $1$ is the one on the periodic table which has the greatest metallic character and group $17$ is the one with the lowest metallic character. These properties include metallic luster, formation of cations, high electrical and thermal conductivity, and malleability. Which element has the least metallic character?

Thus, the elements helium, neon, fluorine,. Which element has the least metallic character? Now if we analyze the trends in the periodic table metallic character decreases as you move from left to right and from bottom to top of the periodic table.
 Source: brainly.com
Source: brainly.com
The right and uppermost elements on the periodic table. Which ion has the largest ionic radius? Which element has the least metallic character?
 Source: xaktly.com
Source: xaktly.com
If we look at the periodic table group 17 and group 18 have the least or lowest metallic character. Which group has least metallic character? When we mmove down the group, the ie decreases, the electropositive character, hence the metallic character also increases.
 Source: slideplayer.com
Source: slideplayer.com
They are located at the right corner uppermost elements. The alkali metals in group 1 are the most active metals, and cesium is the last element in the group for which we have experimental data. This leaves fluorine as the last element, meaning it has the lowest metallic character.
 Source: slidetodoc.com
Source: slidetodoc.com
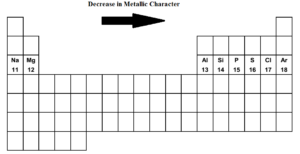
Group 7a, the halogens, show periodic trends with respect to their melting point, boiling point, and density. Of solid and liquid elements at normal temperature and pressure, the least metallic are those at upper right of the periodic table: If we look at the periodic table group 17 and group 18 have the least or lowest metallic character.
 Source: clutchprep.com
Source: clutchprep.com
The alkali metals in group 1 are the most active metals, and cesium is the last element in the group for which we have experimental data. Find answer in image to clear your doubt instantly: What is the least metallic element?
 Source: chegg.com
Source: chegg.com
Thus, the elements helium, neon, fluorine,. Metallic character is mostly dependent on an. For eg helium , neon ,.
 Source: sciencenotes.org
Source: sciencenotes.org
Find answer in image to clear your doubt instantly: If we look at the periodic table group 17 and group 18 have the least or lowest metallic character. Name an element that has 5.
 Source: youtube.com
Source: youtube.com
Group $1$ is the one on the periodic table which has the greatest metallic character and group $17$ is the one with the lowest metallic character. So, fluorine is more electronegative than helium or neon, even though these noble gases are to the right of fluorine on the table. What has the least metallic character?
 Source: slideplayer.com
Source: slideplayer.com
Therefore, the least metallic elements are the opposite: A) si b) al c) na d) mg Chlorine is right below fluorine, making it the element with the fifth highest metallic character.
 Source: thefactfactor.com
Source: thefactfactor.com
The alkali metals in group 1 are the most active metals, and cesium is the last element in the group for which we have experimental data. Sodium fluoride is an ionic compound because it is composed of a positive and negative ion (metal and nonmetal). 3) which element has the least metallic character?* a) sodium (na) b) beryllium (be) c) potassium (k) d) magnesium (mg)
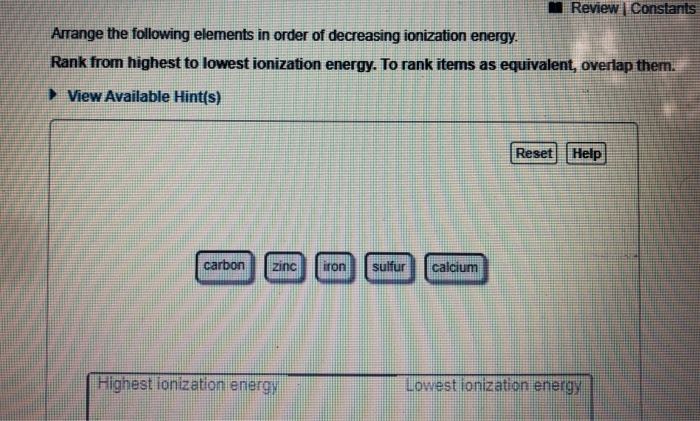 Source: chegg.com
Source: chegg.com
Chlorine is right below fluorine, making it the element with the fifth highest metallic character. The alkali metals in group 1 are the most active metals, and cesium is the last element in the group for which we have experimental data. Halogens near the top of the periodic table are the least metallic elements, not the noble gases.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Therefore, the least metallic elements are the opposite: Halogens near the top of the periodic table are the least metallic elements, not the noble gases. Which is the correct formula for the compound formed from magnesium and sulfur?
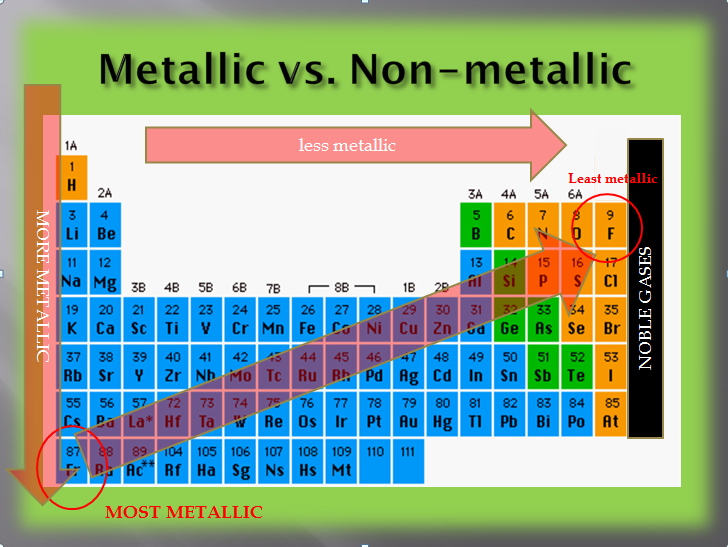 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
Which element has the least metallic character. The elements with the most metallic character are on the left side of the periodic table (except hydrogen). Which group has least metallic character.
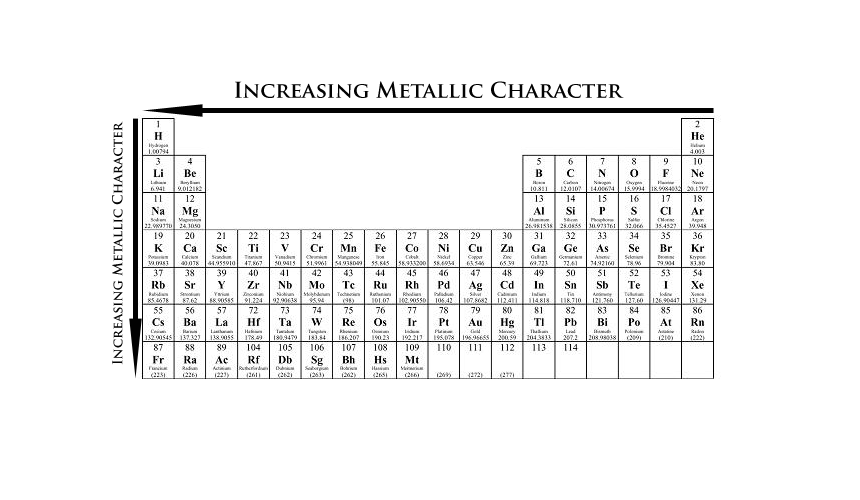 Source: 170188733453308075.weebly.com
Source: 170188733453308075.weebly.com
Carbon, phosphorus, sulfur, bromine and iodine. Which group has least metallic character? A) si b) al c) na d) mg
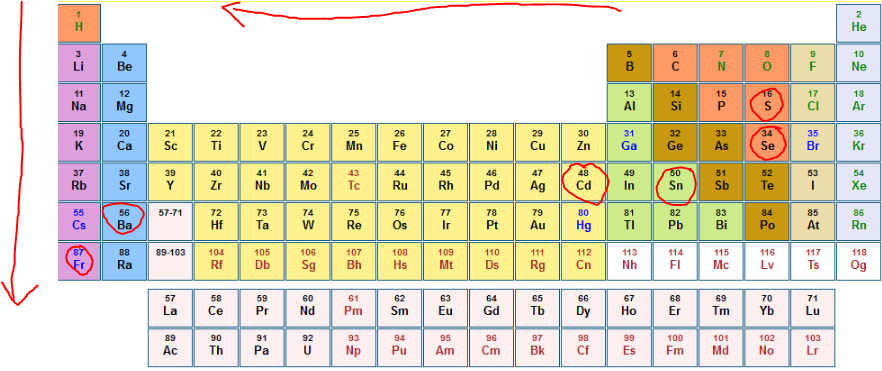 Source: clutchprep.com
Source: clutchprep.com
The least metallic elements is exactly the opposite to metallic elements. What is the least metallic element? If we look at the periodic table group 17 and group 18 have the least or lowest metallic character.
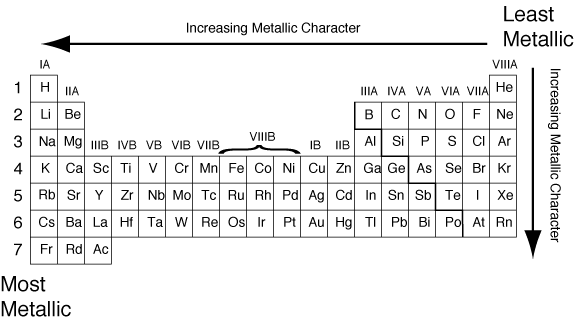 Source: theptproject.weebly.com
Source: theptproject.weebly.com
Which ion has the largest ionic radius? If we look at the periodic table group 17 and group 18 have the least or lowest metallic character. Find answer in image to clear your doubt instantly:
 Source: chemistrybytes.com
Source: chemistrybytes.com
The elements with the most metallic character are on the left side of the periodic table (except hydrogen). Therefore, the least metallic elements are the opposite: Metallic character is the set of properties associated with metals.
Also Read :





