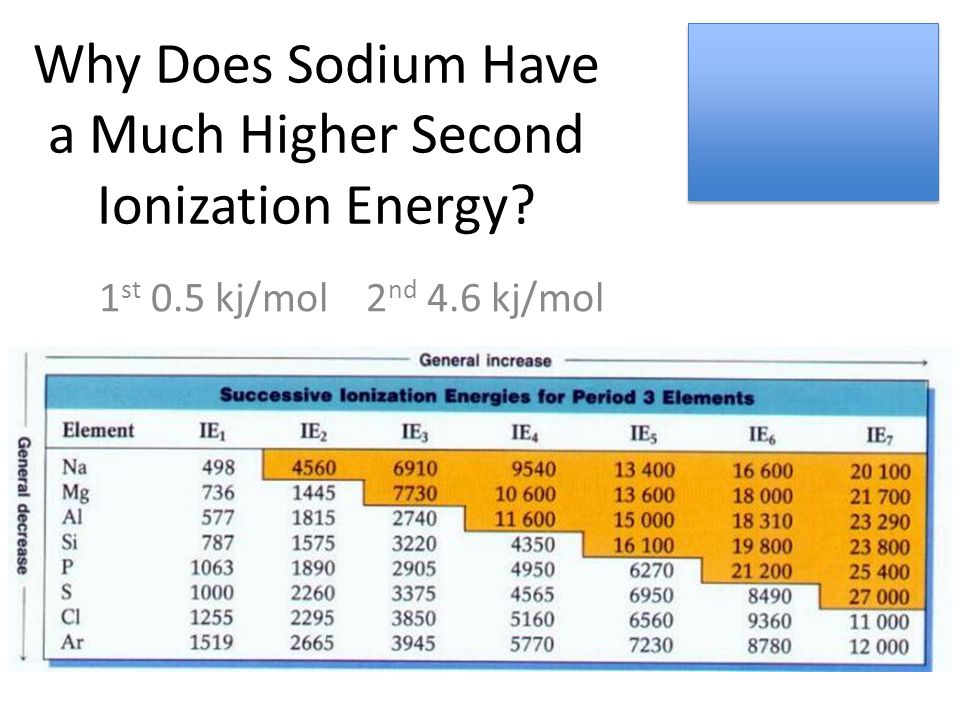
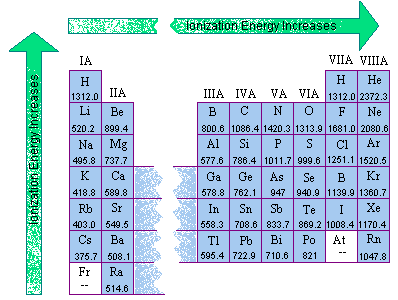
Ionization energies of gaseous atoms (kj/mol) atomic # 1 12 mg 737.8 13 al 577.6 14 si 786.5 15 p 1,012 what element has a first ionization energy of 1000? Li has the highest ie2, because to remove the second electron we must break the stable 1s2 noble gas shell.
Which Element Has The Highest Second Ionization Energy. Neon is maller than argon considering difficult to ionize or remove electrons from neon as compare to argon. Which element listed below would most likely have the. Which one will have the highest 2nd ionization energy? When n a + ions loses an electron, this stable configuration of n e is broken.

Related Post Which Has The Smallest First Ionization Energy? - Quora :
Now sodium and aluminium has achieved its stable conf. E.) is the energy required to remove an electron from a gaseous atom or ion. Refers to removing a second electron. When n a + ions loses an electron, this stable configuration of n e is broken.
E.) is the energy required to remove an electron from a gaseous atom or ion.
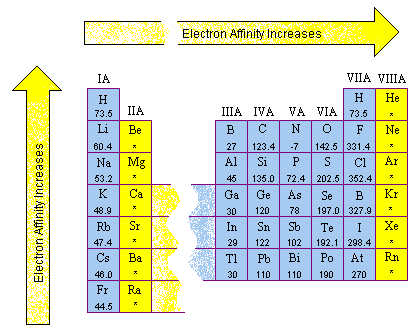
From this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon). Beside above, which group has the highest second ionization energy? All right, so coffer vs gold, which has three high wire ionization energy. Li has the highest ie2 , because to remove the second electron we must break the stable 1s2 noble gas shell. A quick look in the periodic table will reveal that aluminium, al, and magnesium, mg, are both located in period 3. The first or initial ionization energy or ei of an atom or molecule is the energy required to remove one mole of electrons from one mole of isolated gaseous atoms or ions.
 Source: socratic.org
Source: socratic.org
Now, assuming that you�re not familiar with the periodic trends in ionization energy, you can determine which element will have a higher third ionization energy by taking a look at their respective electron configurations. The tabular chart on the right is arranged by ionization energy. Which of the following elements has the largest second ionization energy (ie2)?
 Source: clutchprep.com
Source: clutchprep.com
It is because of the shielding effect that the ionization energy decreases from top to bottom within a group. The third electron comes from a 2p6 and is very difficult to remove. Now, assuming that you�re not familiar with the periodic trends in ionization energy, you can determine which element will have a higher third ionization energy by taking a look at their respective electron configurations.

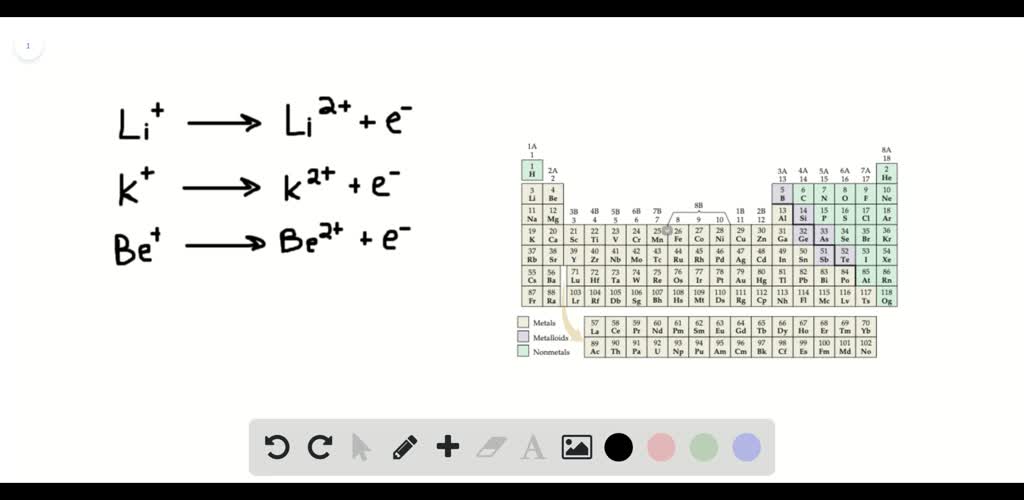
An element’s second ionization energy is the energy required to remove the outermost, or least bound, electron from a 1+ ion of the element. The 2nd ionization energy of the element m is a measure of the energy required to remove one electron from one mole of the gaseous ion m +. When sodium loses one electron, it forms n a + ion which has stable electronic configuration of the noble gas n e.
 Source: slideplayer.com
Source: slideplayer.com
The 2nd ionization energy of the element m is a measure of the energy required to remove one electron from one mole of the gaseous ion m +. Hence, second ionization energy of sodium is largest. The first or initial ionization energy or ei of an atom or molecule is the energy required to remove one mole of electrons from one mole of isolated gaseous atoms or ions.
 Source: youtube.com
Source: youtube.com
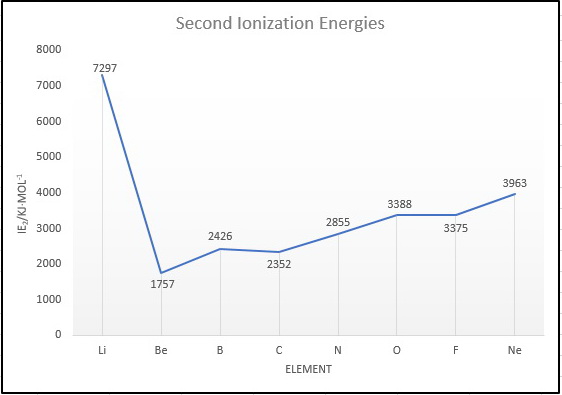
O has a greater ie2 than f. Li has the highest ie2 , because to remove the second electron we must break the stable 1s2 noble gas shell. What element has an ionization energy of 1012?
 Source: youtube.com
Source: youtube.com
Which element in period 3 has highest second ionisation energy? Mg outer electron configuration is 3s2 so the first two electrons are easy to remove. Which element listed below would most likely have the.
 Source: youtube.com
Source: youtube.com
Which element in period 3 has highest second ionisation energy? A quick look in the periodic table will reveal that aluminium, al, and magnesium, mg, are both located in period 3. Which element in period 3 has highest second ionisation energy?
 Source: wps.prenhall.com
Source: wps.prenhall.com
Refers to removing a second electron. Refers to removing a second electron. Why does lithium have the highest second ionisation energy?
 Source: youtube.com
Source: youtube.com
Li has the highest ie2, because to remove the second electron we must break the stable 1s2 noble gas shell. Neon is maller than argon considering difficult to ionize or remove electrons from neon as compare to argon. The 2nd ionization energy of the element m is a measure of the energy required to remove one electron from one mole of the gaseous ion m +.
 Source: chegg.com
Source: chegg.com
Because positive charge binds electrons more strongly, the second ionization energy of an. Why do aluminum and gallium have almost identical 1st ionization energies? Refers to removing a second electron.
 Source: socratic.org
Source: socratic.org
Mg outer electron configuration is 3s2 so the first two electrons are easy to remove. If we were to take a single element then helium is said to have the highest first ionization energy among all the other neutral elements. All right, so coffer vs gold, which has three high wire ionization energy.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
If we were to take a single element then helium is said to have the highest first ionization energy among all the other neutral elements. Thus, helium has the largest first ionization energy, while francium has one of the lowest….periodic trends — […] The unity for ionization energy is ev.
 Source: clutchprep.com
Source: clutchprep.com
Ie1 ie2 ie3 ie4 ie5 ie6 577 kj/mol 1816 kj/mol 2744 kj 11,576 kj/mol 14,829 kj/mol 18,375 kj/mol. Ie1 ie2 ie3 ie4 ie5 ie6 577 kj/mol 1816 kj/mol 2744 kj 11,576 kj/mol 14,829 kj/mol 18,375 kj/mol. A quick look in the periodic table will reveal that aluminium, al, and magnesium, mg, are both located in period 3.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
Now sodium and aluminium has achieved its stable conf. This is probably due to the extra stability of the s2 subshell in the b+ ion. Group viii a have the highest ionization energy but they rarely react.therefore, group vii a elements have the highest ionization energy because it requires a very high amount of energy to remove an electron from halogens as they have 7 electrons in their last orbit.
 Source: clutchprep.com
Source: clutchprep.com
Mg outer electron configuration is 3s2 so the first two electrons are easy to remove. From this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon). Beside above, which group has the highest second ionization energy?
 Source: angelo.edu
Source: angelo.edu
Hence, second ionization energy of sodium is largest. Hence, second ionization energy of sodium is largest. The ion with smaller size can produce stronger attractions on outer electrons which results in high ionization energy.

Ionization energies of gaseous atoms (kj/mol) atomic # 1 12 mg 737.8 13 al 577.6 14 si 786.5 15 p 1,012 what element has a first ionization energy of 1000? This is probably due to the extra stability of the s2 subshell in the b+ ion. Li has the highest ie2 , because to remove the second electron we must break the stable 1s2 noble gas shell.
 Source: chegg.com
Source: chegg.com
Refers to removing a second electron. First, second and third ionization energy (ev) first. Also ionization increases left to right across a period.
 Source: differencebetween.com
Source: differencebetween.com
The first or initial ionization energy or ei of an atom or molecule is the energy required to remove one mole of electrons from one mole of isolated gaseous atoms or ions. Now, assuming that you�re not familiar with the periodic trends in ionization energy, you can determine which element will have a higher third ionization energy by taking a look at their respective electron configurations. Neon is maller than argon considering difficult to ionize or remove electrons from neon as compare to argon.
 Source: numerade.com
Source: numerade.com
Because positive charge binds electrons more strongly, the second ionization energy of an. Which element listed below would most likely have the. Element q is a third period element and has the following successive ionization energies.
Also Read :