Which electron configuration is an atom in an excited state? How many ions are in na?
Which Electron Configuration Is Correct For A Sodium Ion. The alkali metal sodium (atomic. Within the highest energy level, electrons are removed in order of p then s. Which element or ion listed below has the electron configuration 1s22s22p63s23p6? The atom of sodium has 11 electrons, 11 protons along with 12 neutrons, but na+ contains one less electron, 11 protons along with 12 neutrons, as the ion has lost 1 electron.
![Solved:identify The Shorthand Electron Configuration Of A Sodium Ion Na+ Oa [Ne] Ob. [Ar] Oc: [He] Op: 1S22S22P6 1521P6252 Solved:identify The Shorthand Electron Configuration Of A Sodium Ion Na+ Oa [Ne] Ob. [Ar] Oc: [He] Op: 1S22S22P6 1521P6252](https://cdn.numerade.com/ask_images/d19429c76f17406d88a7feb5ff7bb2c4.jpg) Solved:identify The Shorthand Electron Configuration Of A Sodium Ion Na+ Oa [Ne] Ob. [Ar] Oc: [He] Op: 1S22S22P6 1521P6252 From numerade.com
Solved:identify The Shorthand Electron Configuration Of A Sodium Ion Na+ Oa [Ne] Ob. [Ar] Oc: [He] Op: 1S22S22P6 1521P6252 From numerade.com
Related Post Solved:identify The Shorthand Electron Configuration Of A Sodium Ion Na+ Oa [Ne] Ob. [Ar] Oc: [He] Op: 1S22S22P6 1521P6252 :
One electron is lost to form the sodium ion. The p orbital can hold up to six electrons. Write the electron configuration for sodium. Which element or ion listed below has the electron configuration 1s22s22p63s23p6?
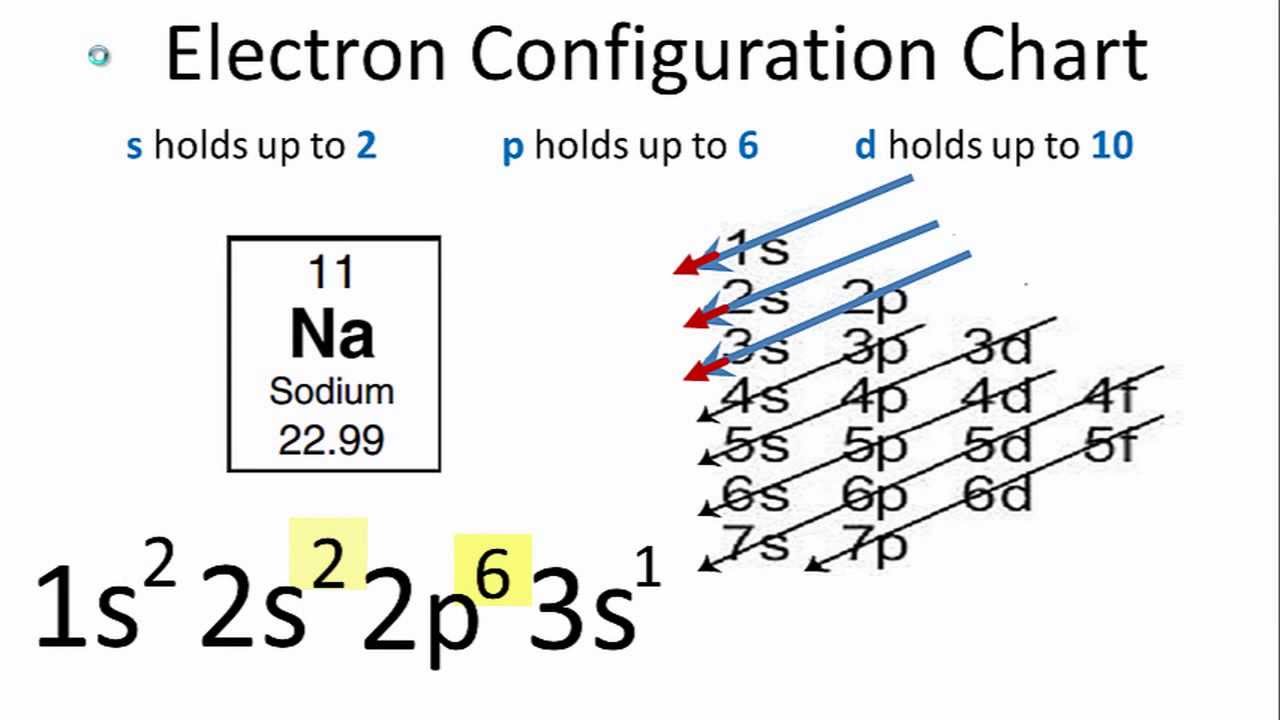
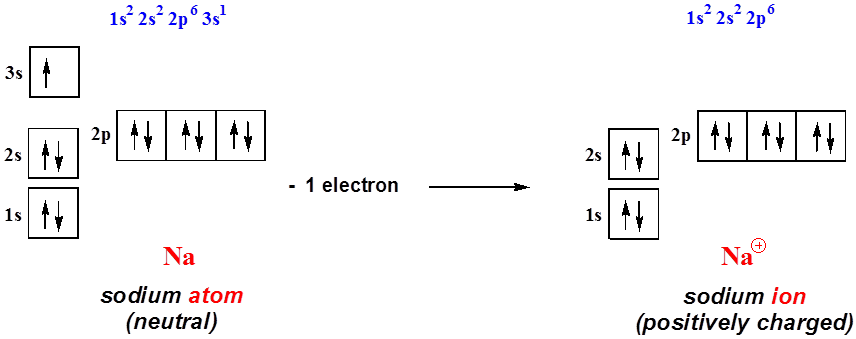
The electron configuration of a neutral sodium atom is #1s^2 2s^2 2p^6 3s^1#.
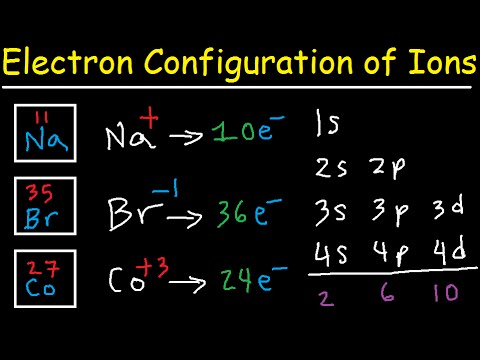
Finally, what is the electron configuration for an mg2+ ion?, therefore. The overall number of electrons is now 18. We�ll put six in the 2p orbital and then put the remaining electron in the 3s. The electron configuration shows the distribution of electrons into subshells. Which electron configuration is an atom in an excited state? The total number of electrons is 11 in the sodium atom.
 Source: terpconnect.umd.edu
Source: terpconnect.umd.edu
Within the highest energy level, electrons are removed in order of p then s. The first is done for you wrong electron configuration correct electron configuration a 1s 2 2s 2 3s 2 3p 2 1s 2 2s 2 2p 4 this is the correct way to do 8 b 1s 2 2s 2 2p 5 3s. The n shell containing 4s, 4d,.
 Source: periodictable.me
Source: periodictable.me
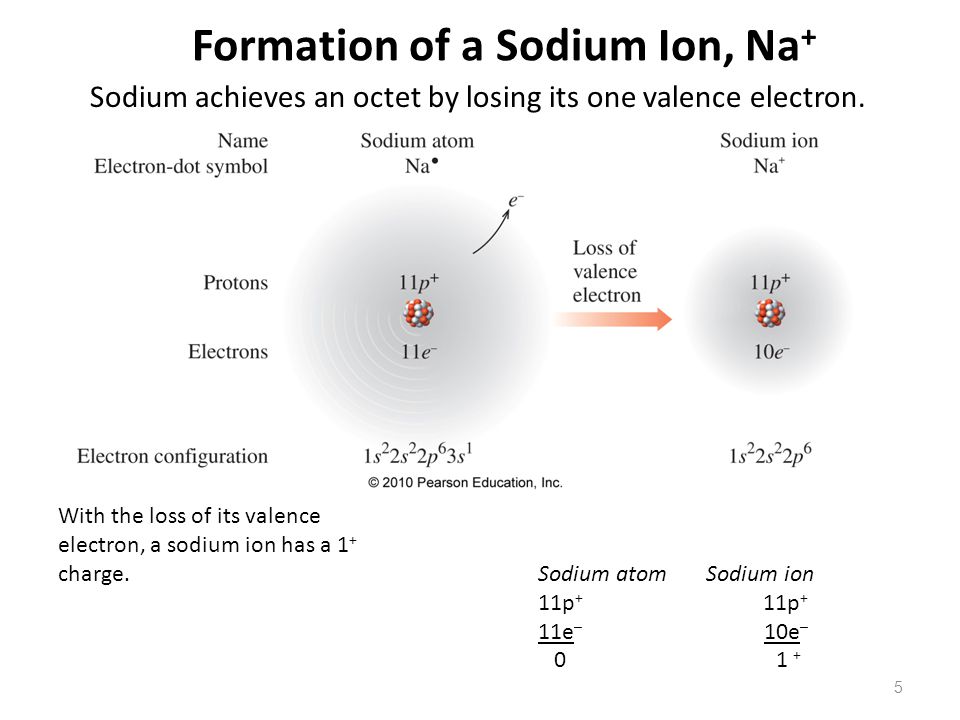
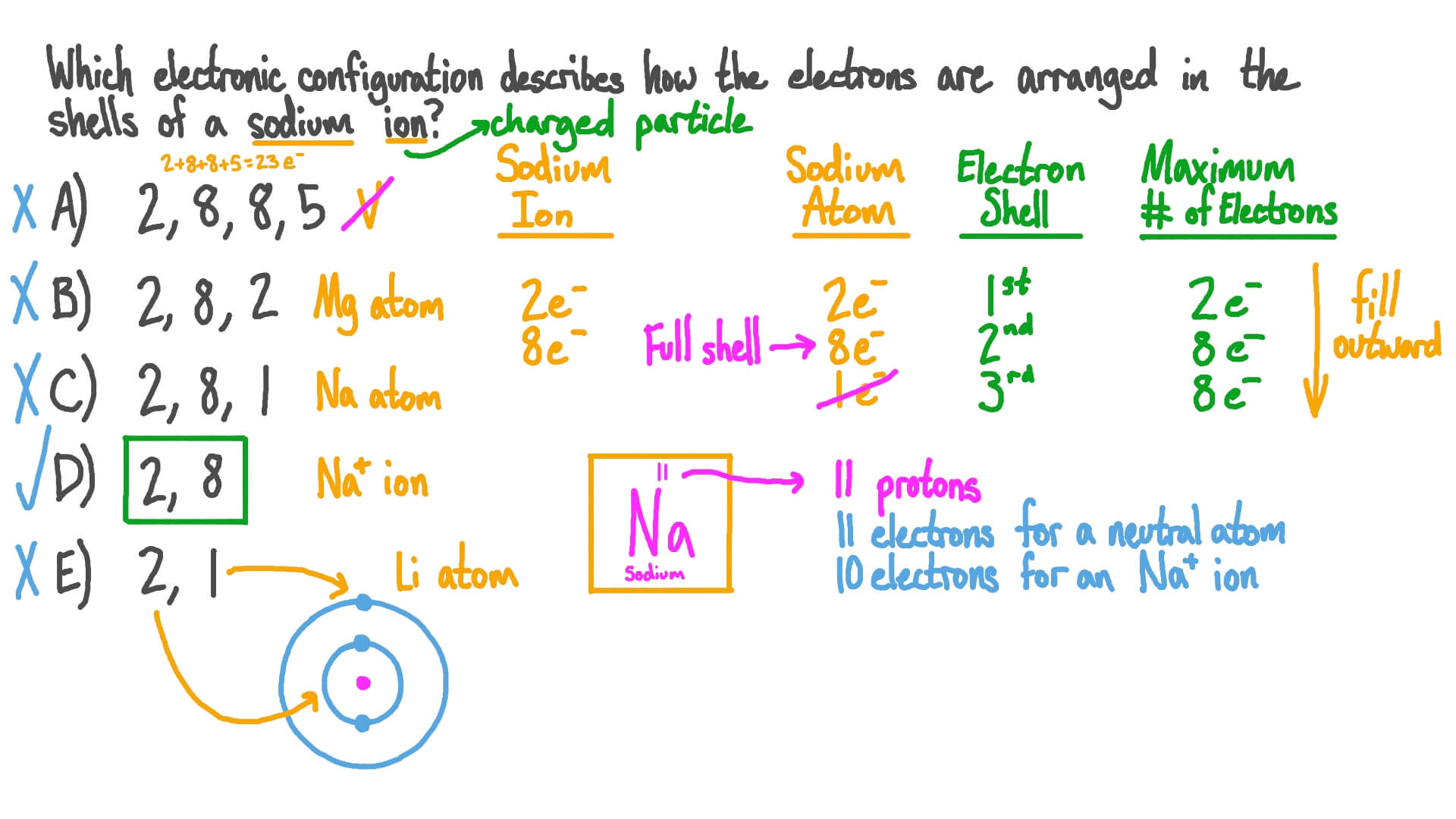
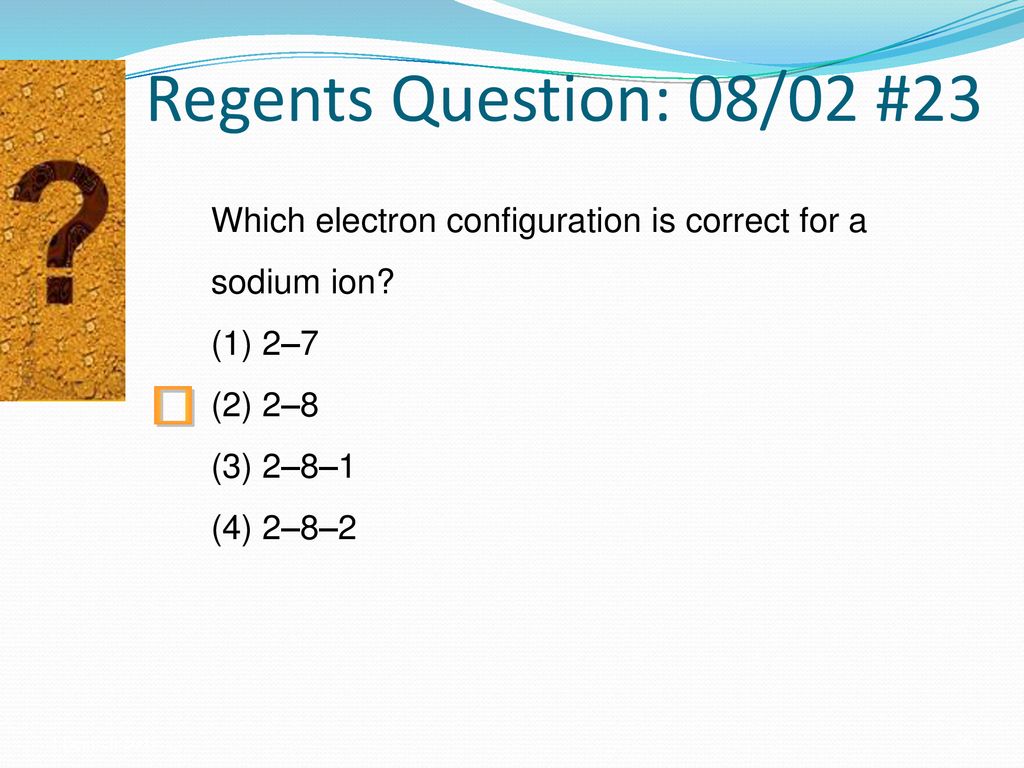
The electronic configuration of sodium is 2, 8, 1. (2) (e) a sample of xenon has a r = 131.31. We’ll also look at why sodium forms a 1+ ion and how the electron configurati.
 Source: nagwa.com
Source: nagwa.com
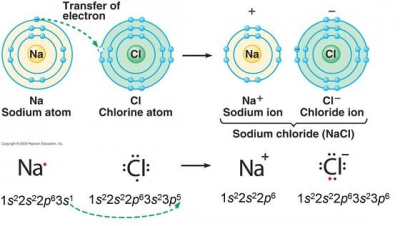
All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Na + cl ¨ na+ + cl− ¨ nacl. The p orbital can hold up to six electrons.
 Source: periodictable.me
Source: periodictable.me
The m shell contains 3s, 3p, and 3d, and can carry 18 electrons. The sample consists of four. These ions are then attracted to each other in a 1:1 ratio to form sodium chloride (nacl).
 Source: docbrown.info
Source: docbrown.info
What is the electron configuration notation for sodium? When forming a cation, the electron is removed from the energy level with the highest n value. 1s2 2s2 2p6 3p1 b.
 Source: socratic.org
Source: socratic.org
[ne] 3s^2 3p^6 chemistry science Write the electron configuration for sodium. We’ll also look at why sodium forms a 1+ ion and how the electron configurati.
 Source: slideplayer.com
Source: slideplayer.com
The total number of electrons is 11 in the sodium atom. How do you write the electron configuration for magnesium?, the electron configuration for magnesium is 1s 2 2s 2 2p 6 3s 2. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4.
 Source: socratic.org
Source: socratic.org
[ne] 3s^2 3p^6 chemistry science [ne] 3s^2 3p^6 chemistry science We’ll also look at why sodium forms a 1+ ion and how the electron configurati.
![Solved:identify The Shorthand Electron Configuration Of A Sodium Ion Na+ Oa [Ne] Ob. [Ar] Oc: [He] Op: 1S22S22P6 1521P6252 Solved:identify The Shorthand Electron Configuration Of A Sodium Ion Na+ Oa [Ne] Ob. [Ar] Oc: [He] Op: 1S22S22P6 1521P6252](https://cdn.numerade.com/ask_images/d19429c76f17406d88a7feb5ff7bb2c4.jpg) Source: numerade.com
Source: numerade.com
In the case of sodium the one lone electron in the 3s valence shell would easily be released in order for sodium to have a filled valence shell at 2s22p6. It will contain 6.022 x 10 23 sodium ions and 6.022 x 10 23 chloride ions. What is the electron configuration notation for sodium?

The electron configuration shows the distribution of electrons into subshells. For atomic br, we have z = 35, and thus for br− we gots 36 electrons to distribute in the usual way. We’ll also look at why sodium forms a 1+ ion and how the electron configurati.
 Source: slideplayer.com
Source: slideplayer.com
Thus, its electron configuration is: What is the electron configuration notation for sodium? It will contain 6.022 x 10 23 sodium ions and 6.022 x 10 23 chloride ions.
 Source: slidetodoc.com
Source: slidetodoc.com
One electron is lost to form the sodium ion. To save room, the configurations are in noble gas shorthand.this means part of the electron configuration has been replaced with the element symbol of the noble gas symbol. The first is done for you wrong electron configuration correct electron configuration a 1s 2 2s 2 3s 2 3p 2 1s 2 2s 2 2p 4 this is the correct way to do 8 b 1s 2 2s 2 2p 5 3s.
 Source: youtube.com
Source: youtube.com
This give us the (correct) configuration of: [ne] 3s^2 3p^6 chemistry science The electronic configuration of sodium is 2, 8, 1.
 Source: slideserve.com
Source: slideserve.com
When sodium (na) and chlorine (cl) are combined, the sodium atoms each lose an electron, forming cations (na+), and the chlorine atoms each gain an electron to form anions (cl−). 1s2 2s2 2p6 3s2 3p6 3d5 4s1. The electron configuration of a neutral sodium atom is 1 s2 2 s2 2 p6 3 s1.
 Source: chemistryfromscratch.org
Source: chemistryfromscratch.org
The alkali metal sodium (atomic. Which element or ion listed below has the electron configuration 1s22s22p63s23p6? All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled.
 Source: slidetodoc.com
Source: slidetodoc.com
The atomic number of sodium is 11. We’ll also look at why calcium forms a 2+ ion and how the electron configur. The electronic configuration of sodium is 2, 8, 1.
 Source: slideplayer.com
Source: slideplayer.com
The p orbital can hold up to six electrons. Na + cl ¨ na+ + cl− ¨ nacl. The nex six electrons will go in the 2p orbital.
 Source: slidetodoc.com
Source: slidetodoc.com
Which element or ion listed below has the electron configuration 1s22s22p63s23p6? The electron configuration of a neutral sodium atom is 1 s2 2 s2 2 p6 3 s1. [ne] 3s^2 3p^6 chemistry science
 Source: youtube.com
Source: youtube.com
The n shell containing 4s, 4d,. Therefore , the electron configuration of the sodium ion is. The electron configuration of a neutral sodium atom is #1s^2 2s^2 2p^6 3s^1#.
 Source: youtube.com
Source: youtube.com
We’ll also look at why sodium forms a 1+ ion and how the electron configurati. The first is done for you wrong electron configuration correct electron configuration a 1s 2 2s 2 3s 2 3p 2 1s 2 2s 2 2p 4 this is the correct way to do 8 b 1s 2 2s 2 2p 5 3s. The k shell contains a 1s subshell hence it can carry 2 electrons, the l shell has 2s and 2p, and can carry 8 electrons.
Also Read :