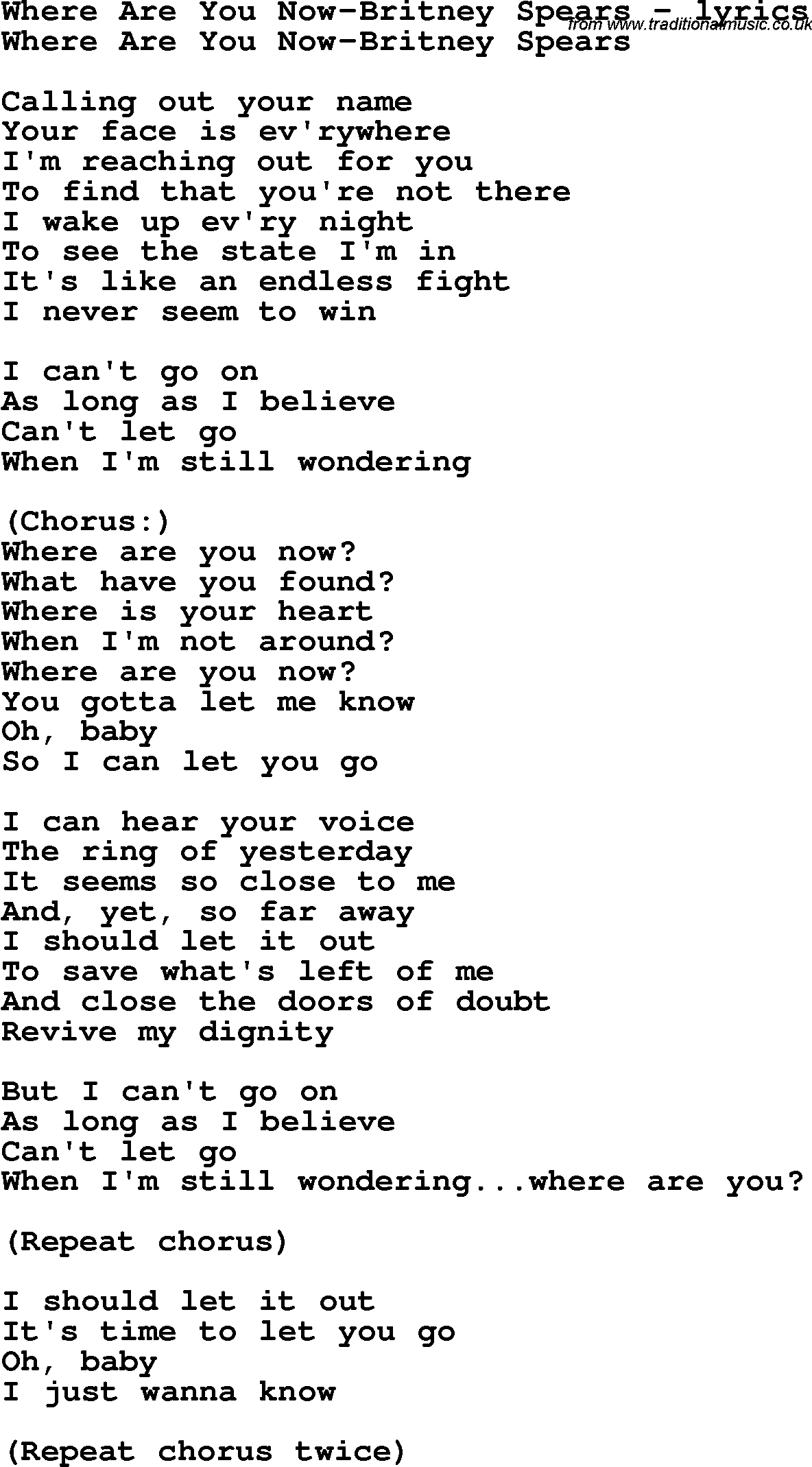
As a result, solutions containing nonelectrolytes will not conduct electricity. The molecular weight of water is 18.0 g/mol.
Which Compound Is A Nonelectrolyte. When these compounds dissolve in water , they do not produce ions. 3.the molecular weight of the compound. The definition of electrolyte is a substance which forms ion in an aqueous solution. They retain their molecular structure.
 Solved:identify Each Of The Following Compounds As A Nonelectrolyte, A Weak Electrolyte, Or A Strong Electrolyte: (A) Ethanolamine \Left(\Mathrm{C}{2} \Mathrm{H}{5} \Mathrm{Onh}_{2}\Right), (B) Potassium Fluoride (Kf), (C) Ammonium Nitrate \Left … From numerade.com
Solved:identify Each Of The Following Compounds As A Nonelectrolyte, A Weak Electrolyte, Or A Strong Electrolyte: (A) Ethanolamine \Left(\Mathrm{C}{2} \Mathrm{H}{5} \Mathrm{Onh}_{2}\Right), (B) Potassium Fluoride (Kf), (C) Ammonium Nitrate \Left … From numerade.com
Related Post Solved:identify Each Of The Following Compounds As A Nonelectrolyte, A Weak Electrolyte, Or A Strong Electrolyte: (A) Ethanolamine \Left(\Mathrm{C}{2} \Mathrm{H}{5} \Mathrm{Onh}_{2}\Right), (B) Potassium Fluoride (Kf), (C) Ammonium Nitrate \Left … :
1.the density of the solute. When these compounds dissolve in water, they do not produce ions. Those that follow the rules of being soluble in solubility rules b. Many molecular compounds, such as sugar or ethanol , are nonelectrolytes.
This question needs to be more focused.
A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. One of these compounds is an electrolyte, meaning that it. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. The answer is (3) hcl. A strong electrolyte completely ionizes when dissolved in water. Those that follow the rules of being soluble in solubility rules b.
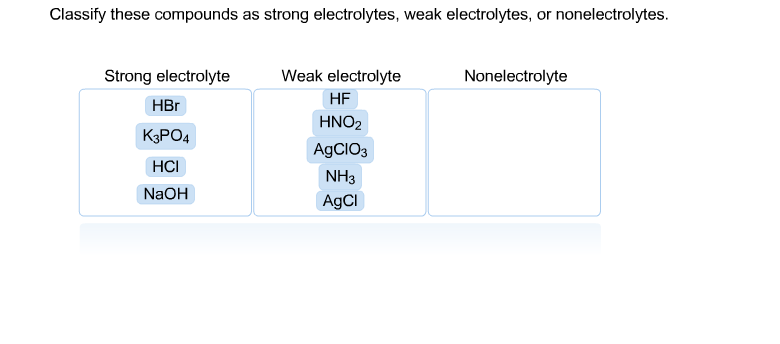 Source: chegg.com
Source: chegg.com
Is acetic acid a nonelectrolyte? Many molecular compounds, such as sugar or ethanol, are nonelectrolytes. For example, glucose is a nonelectrolyte and does not disintegrate in a solvent.
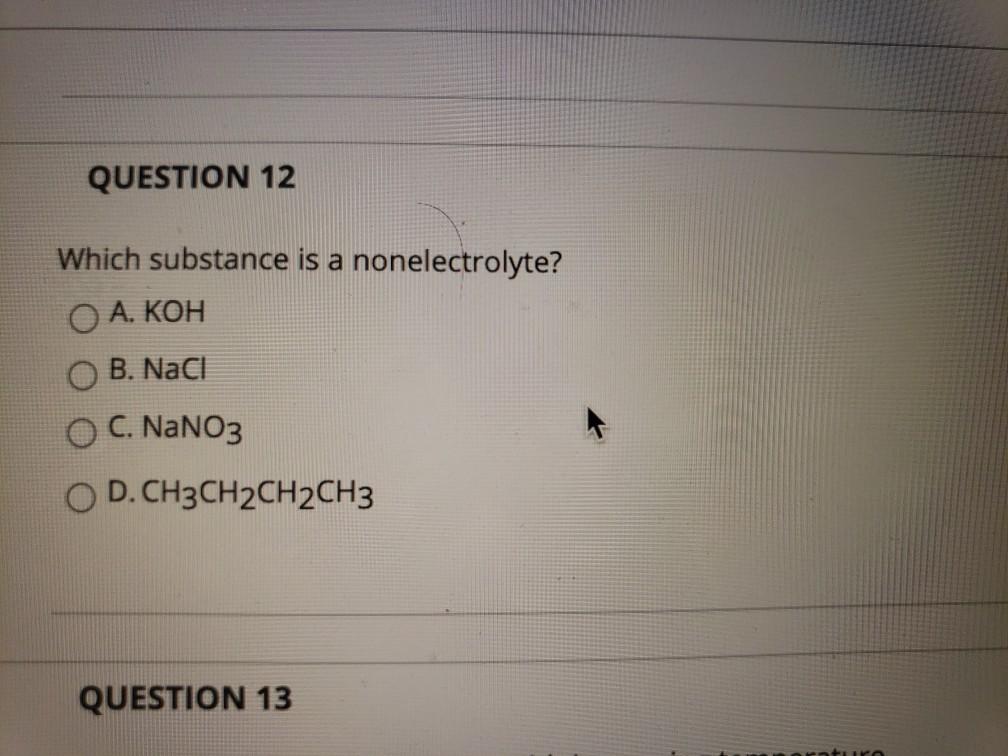
This phenomenon is also the reason why solutions containing sugar do not conduct electricity. A strong electrolyte completely ionizes when dissolved in water. They are compounds that do not disintegrate in polar solvents.
 Source: wps.prenhall.com
Source: wps.prenhall.com
1.the density of the solute. Strong electrolyte weak electrolyte nonelectrolyte. Conduction of electricity in liquids
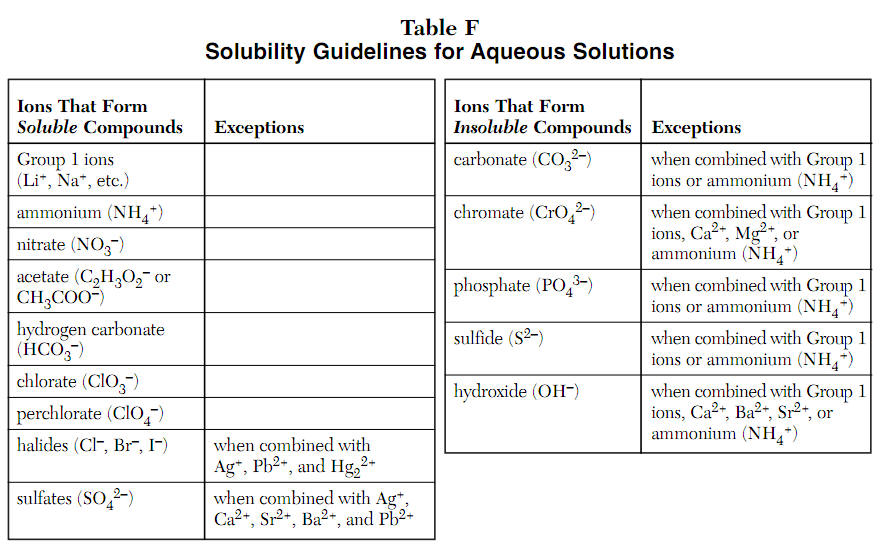 Source: kentchemistry.com
Source: kentchemistry.com
Acetic acid (ch 3 cooh), the compound in vinegar, is a weak electrolyte. We’re being asked to classify each given compound as a strong electrolyte, weak electrolyte, or nonelectrolytes.recall that: One of these compounds is an electrolyte, meaning that it.
 Source: clutchprep.com
Source: clutchprep.com
Electrolytes are also classified by the charges on the ions they form when dissolved in water. The mole fraction of a certain nonelectrolyte compound in a solution containing only that substance and water is 0.100. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution.
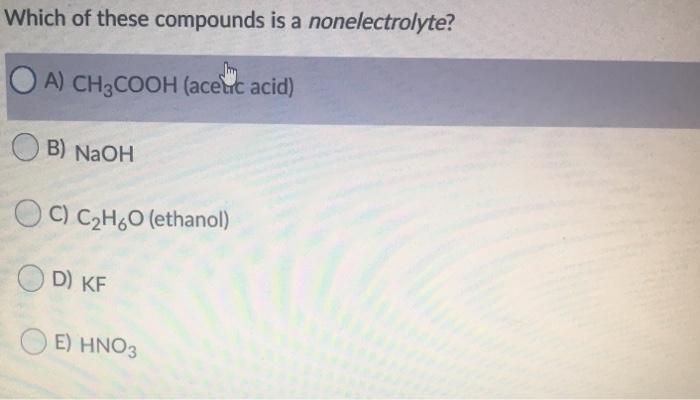
A solution containing 6g of the compound exerts the same osmotic pressure as that of 0.05 m glucose solution at the same temperature. Viewed 939 times 2 $\begingroup$ closed. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state.
 Source: chegg.com
Source: chegg.com
For example, glucose is a nonelectrolyte and does not disintegrate in a solvent. This phenomenon is also the reason why solutions containing sugar do not conduct electricity. When these compounds dissolve in water, they do not produce ions.
 Source: brainly.com
Source: brainly.com
A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. Now up your study game with learn mode. When these compounds dissolve in water , they do not produce ions.
 Source: clutchprep.com
Source: clutchprep.com
The mole fraction of a certain nonelectrolyte compound in a solution containing only that substance and water is 0.100. Nacl (solution) is an electrolyte in the molten. Sodium chloride is an ionic compound that is made up of the sodium cation and the chloride anion.
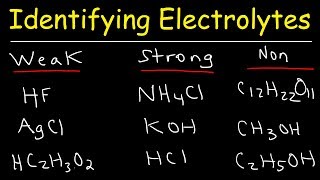 Source: youtube.com
Source: youtube.com
Now up your study game with learn mode. Many molecular compounds, such as sugar or ethanol, are nonelectrolytes. Solutes that dissolve into individual neutral molecules without dissociation do not impart additional electrical conductivity to their solutions and are called nonelectrolytes a compound that does not ionize at all when it dissolves.
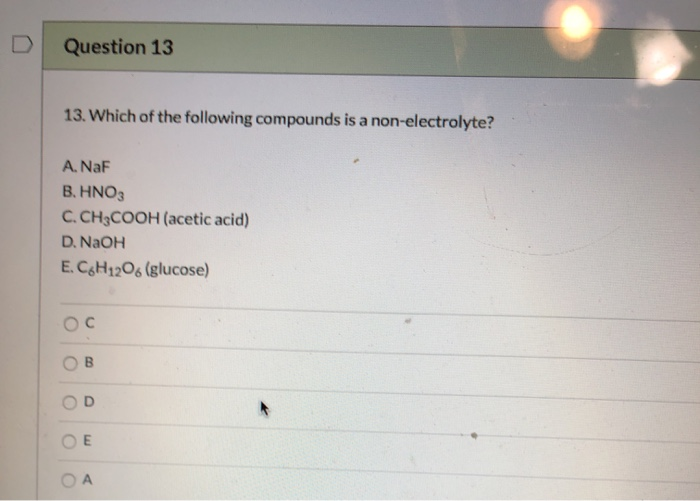 Source: chegg.com
Source: chegg.com
A common example of a nonelectrolyte is glucose, or c6h12o6. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. Nonelectrolyte solutions do not, therefore, conduct electricity.
 Source: slideplayer.com
Source: slideplayer.com
A solution containing 6g of the compound exerts the same osmotic pressure as that of 0.05 m glucose solution at the same temperature. Which substance is an electrolyte ccl4? Sodium chloride is an ionic compound that is made up of the sodium cation and the chloride anion.
 Source: focuskimia.com
Source: focuskimia.com
For example, glucose is a nonelectrolyte and does not disintegrate in a solvent. Active 2 years, 3 months ago. For example, carbon tetrachloride ccl4 when dissolved in water does not dissociate into ions and therefore, it does not conduct electricity.
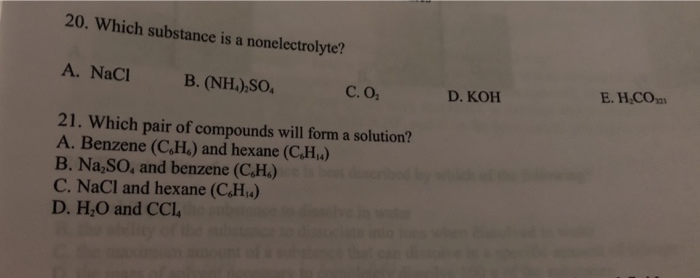 Source: chegg.com
Source: chegg.com
No, nonelectrolytes are compounds formed through covalent bonds; Strong acids and strong bases are strong electrolytes [e.g., hcl(aq), h 2 so 4 (aq), hclo 4 (aq); One of these compounds is an electrolyte, meaning that it.
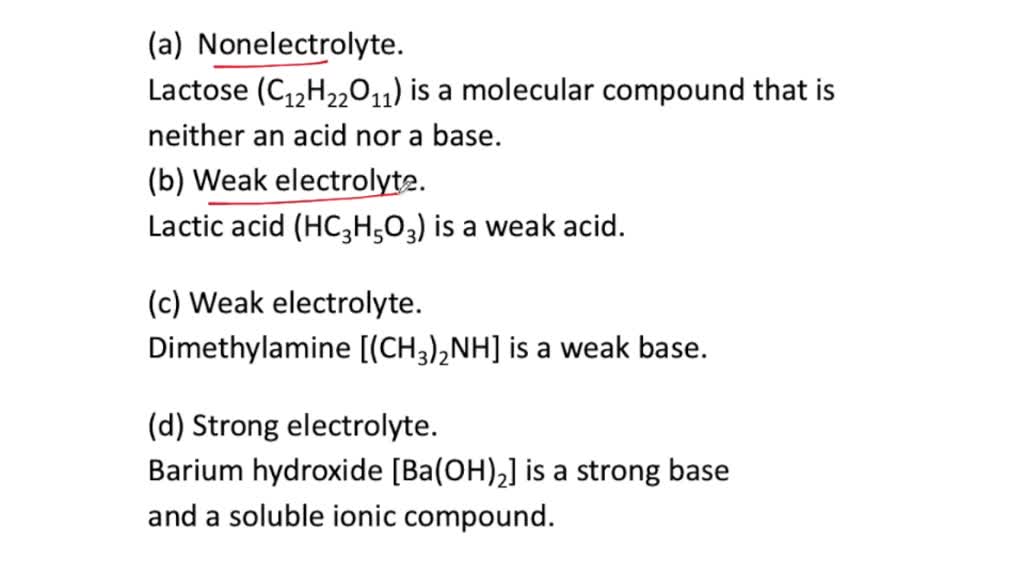 Source: numerade.com
Source: numerade.com
Some examples are oxygen, o2, ethanol, c2h5oh, and sugar, c12h22o11. Classify each compound as a strong electrolyte, weak electrolyte, or nonelectrolyte. Solutes that dissolve into individual neutral molecules without dissociation do not impart additional electrical conductivity to their solutions and are called nonelectrolytes a compound that does not ionize at all when it dissolves.
 Source: chegg.com
Source: chegg.com
- kcl (aq) 2) h2so 4 (dil) 3) ccl 4 (l) 4) ch 3 cooh (aq) class 10 icse. We’re being asked to classify each given compound as a strong electrolyte, weak electrolyte, or nonelectrolytes.recall that: When these compounds dissolve in water , they do not produce ions.
 Source: numerade.com
Source: numerade.com
Now up your study game with learn mode. Strong acids and strong bases are strong electrolytes [e.g., hcl(aq), h 2 so 4 (aq), hclo 4 (aq); They share electron pairs to form such bonds.
 Source: numerade.com
Source: numerade.com
It does not provide ions in a solution and therefore current does not flow through such solution. The molecular formula of the compound is: Classify each compound as a strong electrolyte, weak electrolyte, or nonelectrolyte.
 Source: theengineeringknowledge.com
Source: theengineeringknowledge.com
A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. Conduction of electricity in liquids Typically, nonelectrolytes are primarily held together by covalent rather than ionic bonds.
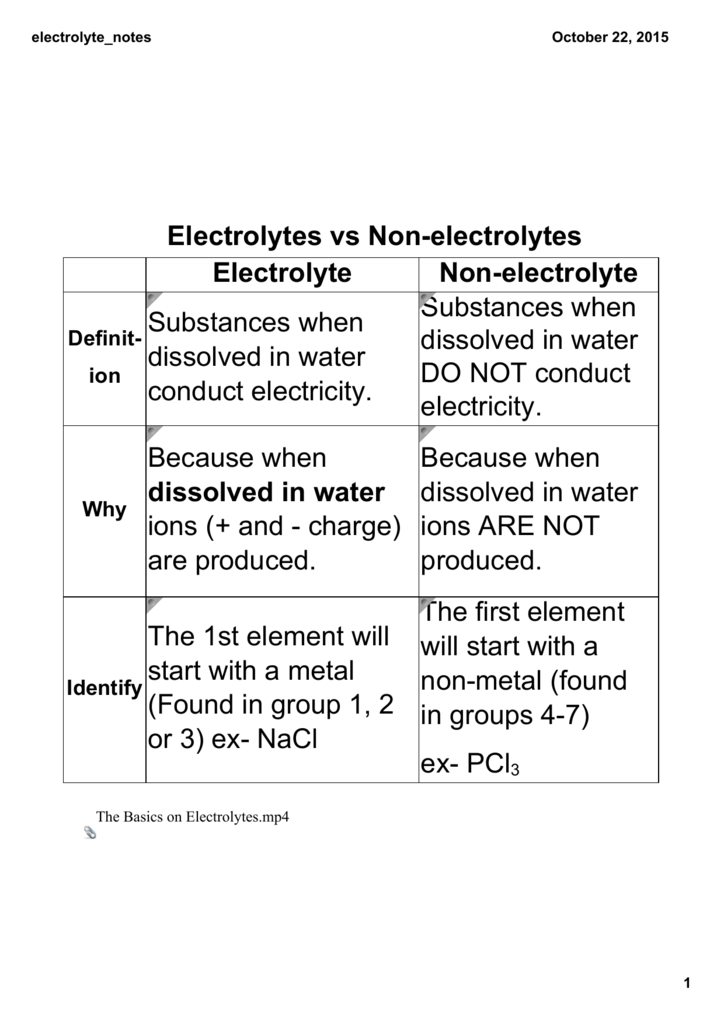 Source: studylib.net
Source: studylib.net
Include soluble ionic salts, strong acids, and bases a. 2.the density of the solution. Usually it is an ionic compound.
Also Read :