The metals in group ia form compounds (such as li 3 n and na 2 s) in which the metal atom has an oxidation number of +1. Yes you are right sulphur has the highest oxidation number.
Which Compound Has The Atom With The Highest Oxidation Number. Casâ na3n mgso3â al(no2)3 nh4cl 2.)â what name (label) is given to the hybridized orbitals of sf6. Manganese, which is in the middle of the period, has the highest number of oxidation states, and indeed the highest oxidation state in the whole period. 1 / 1 pts question 3 which compound has the atom with the highest oxidation number? Group vii elements form highest oxidation numbers.
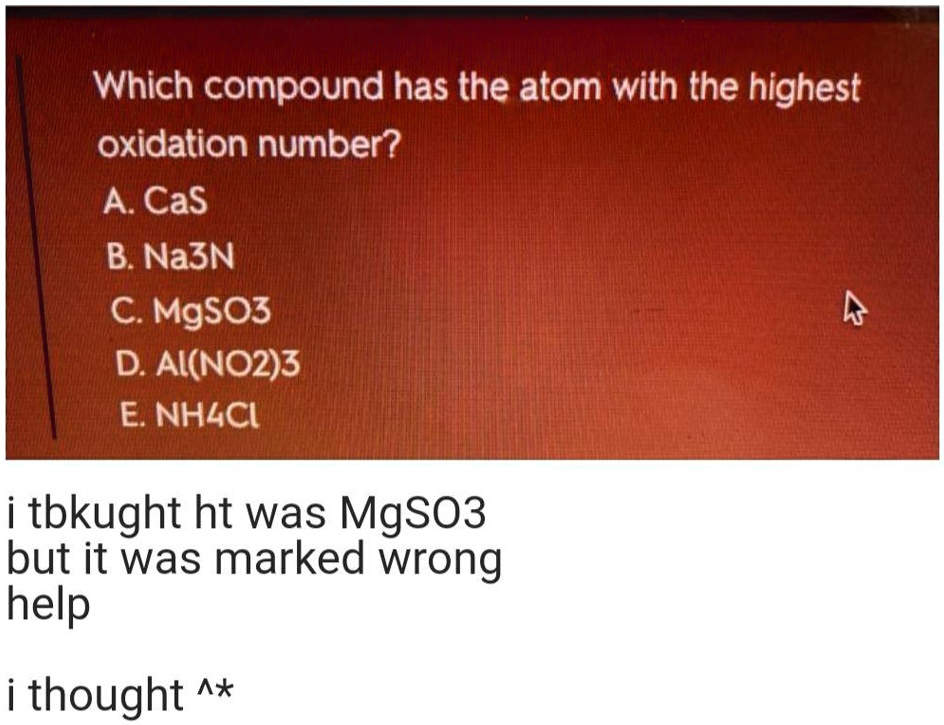 Solved:which Compound Has The Atom With The Highest Oxidation Number? A Cas B. Na3N C Mgso3 D Ai(No2)3 E: Nhlci 1 Tbkught Ht Was Mgso3 But It Was Marked Wrong Help 1 From numerade.com
Solved:which Compound Has The Atom With The Highest Oxidation Number? A Cas B. Na3N C Mgso3 D Ai(No2)3 E: Nhlci 1 Tbkught Ht Was Mgso3 But It Was Marked Wrong Help 1 From numerade.com
Related Post Solved:which Compound Has The Atom With The Highest Oxidation Number? A Cas B. Na3N C Mgso3 D Ai(No2)3 E: Nhlci 1 Tbkught Ht Was Mgso3 But It Was Marked Wrong Help 1 :
Which one of the following has the highest oxidation number of iodine? Manganese has the highest oxidation state because the number of unpaired electrons in the outermost shell is more, i.e. Cas na n 3 mgso correct! View solution > the pair of compounds having metals in their highest oxidation state is:
93% (15 ratings) transcribed image text:
1.) â which compound has the atom wih the highest oxidation number. Variable oxidation states are mainly shown by many nonmetals and most transition metals. Clearly, each atom in h 2, cl 2, p 4, na, al, o 2, o 3, s 8, and mg, has an oxidation number zero. Atoms chemical kinetics moving charges and magnetism microbes in human welfare. The metals in group ia form compounds (such as li 3 n and na 2 s) in which the metal atom has an oxidation number of +1. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons.
 Source: clutchprep.com
Source: clutchprep.com
K i o 4 medium. The nitrogen have oxidation of no2. Clearly, each atom in h 2, cl 2, p 4, na, al, o 2, o 3, s 8, and mg, has an oxidation number zero.
 Source: chegg.com
Source: chegg.com
Which element in a compound has the highest oxidation state? To calculate oxidation numbers of elements in the chemical compound, enter it�s formula and click �calculate� (for example: 1.) â which compound has the atom wih the highest oxidation number.
 Source: chegg.com
Source: chegg.com
Mgso4 what volume of a concentrated solution of sodium hydroxide 6.00must be diluted to 200.0ml to make a 0.880 m solution of sodium hydroxide? Alkali metals (group 1 metals) like. By checking which atom is bonded to elements more electronegative than it is, and generally the atom with the highest oxidation number takes a central position in the molecule.

1.) â which compound has the atom wih the highest oxidation number. 3 al(no ) 2 3 nh cl 4 All alkali metals in the compound form will have oxidation number +1.
 Source: chegg.com
Source: chegg.com
All alkali metals in the compound form will have oxidation number +1. Mgso4 what volume of a concentrated solution of sodium hydroxide 6.00must be diluted to 200.0ml to make a 0.880 m solution of sodium hydroxide? Yes you are right sulphur has the highest oxidation number.
 Source: chegg.com
Source: chegg.com
By checking which atom is bonded to elements more electronegative than it is, and generally the atom with the highest oxidation number takes a central position in the molecule. Yes you are right sulphur has the highest oxidation number. The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds.
 Source: youtube.com
Source: youtube.com
Manganese, which is in the middle of the period, shows the most number of oxidation states, and indeed the highest oxidation state in the whole period since it has five unpaired electrons. Casâ na3n mgso3â al(no2)3 nh4cl 2.)â. 1.) â which compound has the atom wih the highest oxidation number.
 Source: chegg.com
Source: chegg.com
Sulfur has highest oxidation number for. K i o 4 medium. 93% (15 ratings) transcribed image text:
 Source: chegg.com
Source: chegg.com
Which compound has the atom with the highest oxidation number? K i o 4 medium. The metals in group ia form compounds (such as li 3 n and na 2 s) in which the metal atom has an oxidation number of +1.
 Source: clutchprep.com
Source: clutchprep.com
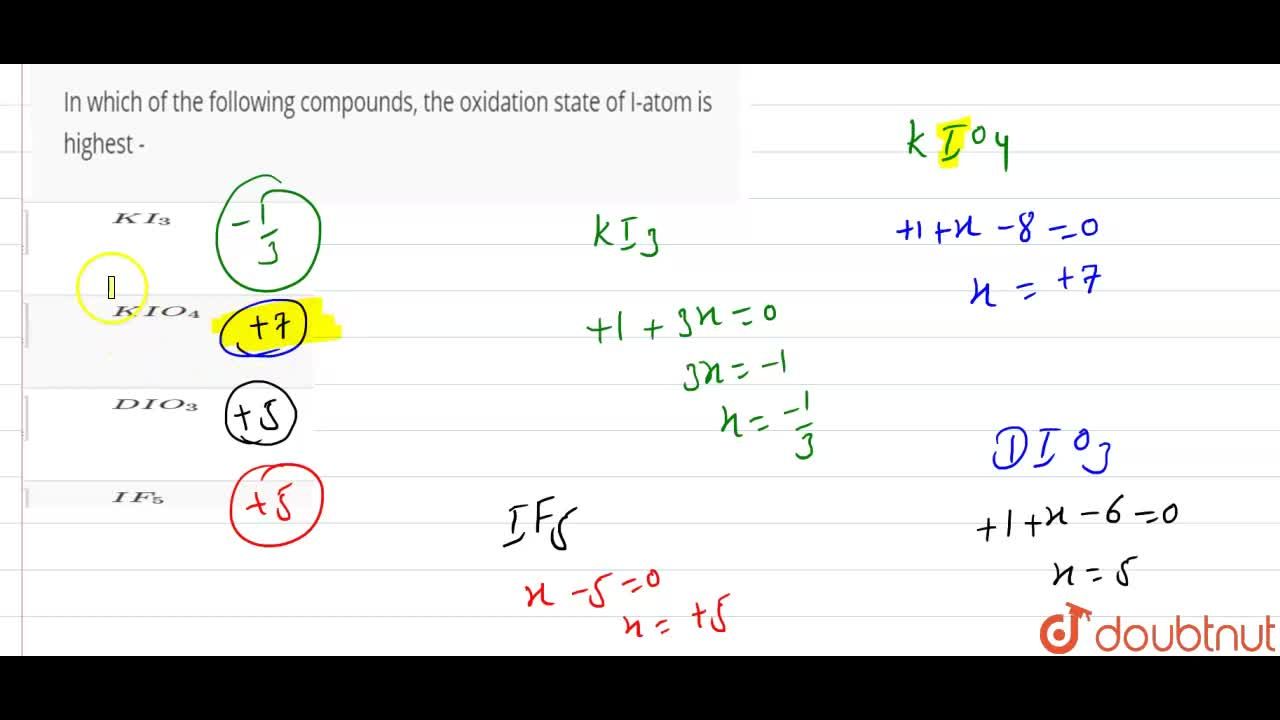
Which element in a compound has the highest oxidation state? Correct option is d) among the given compound, i shows the highest oxidation state and it is + 7. From group vii, chlorine form +7 oxidation number.
 Source: chegg.com
Source: chegg.com
Which one of the following has the highest oxidation number of iodine? K i o 4 medium. To calculate oxidation numbers of elements in the chemical compound, enter it�s formula and click �calculate� (for example:
 Source: doubtnut.com
Source: doubtnut.com
Which elements have the +7 oxidation number and give examples? Which elements have the +7 oxidation number and give examples? In respect to this, which compound has the atom with the highest oxidation number?
 Source: chegg.com
Source: chegg.com
Which compound has the atom with the highest oxidation number? 1 / 1 pts question 3 which compound has the atom with the highest oxidation number? Compound in which the oxidation number of nitrogen is +1 is :

Which compound has the atom with the highest oxidation number?a. Hcooh has highest oxidation state. The total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom.
 Source: numerade.com
Source: numerade.com
- which compound has the atom with the highest oxidation number a) na3n b) ai (no2)3 c) nh4c d) cas e) mgso3. The metals in group ia form compounds (such as li 3 n and na 2 s) in which the metal atom has an oxidation number of +1. Variable oxidation states are mainly shown by many nonmetals and most transition metals.

3 al(no ) 2 3 nh cl 4 The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. Cas na n 3 mgso correct!
 Source: youtube.com
Source: youtube.com
Which element in a compound has the highest oxidation state? Experts are tested by chegg as specialists in their subject area. The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds.

Which compound has the atom with the highest oxidation number?a. Electronegativity is basically how strongly that element draws electrons to itself. Oxidation number of carbon in hcooh= 12×2−2.
 Source: numerade.com
Source: numerade.com
Oxidation number of carbon in hcho= 12×1−2. The oxidation number of ions which comprise of only one atom is equal to the actual charge on the ion. Which one of the following has the highest oxidation number of iodine?
 Source: numerade.com
Source: numerade.com
Atoms chemical kinetics moving charges and magnetism microbes in human welfare semiconductor electronics: Group vii elements form highest oxidation numbers. Ions having one atom bear oxidation number equal to the charge present on the ion.
Also Read :