Why is it true to say that calcium carbonate has both ionic and covalent bonds? So na2co3 has both ionic and covalent bonds.
Which Compound Has Both Ionic And Covalent Bonds. (1) caco3 is the correct answer. Comparison of ionic and covalent bonds ionic bonds usually occur between. For the first part of the question, nh4cl contains both an iconic and covalent bond.the ammonium ion is polyatomic, which means it forms ionic salts. Calcium carbonate is another example of a compound with both ionic and covalent bonds.
 Which Of The Following Compounds Contains Both Ionic … From clutchprep.com
Which Of The Following Compounds Contains Both Ionic … From clutchprep.com
Related Post Which Of The Following Compounds Contains Both Ionic … :
In other words, each ion contains more than one atoms. Caf2 is an ionic compound because, in the caf2 molecule, calcium acts as a cation by donating its 2 extra electrons from the valence shell, and fluoride acts as an anion by accepting the electrons from calcium, completing its octet. Right here calcium acts because the cation, with the carbonate species because the anion. Which kinds of bonds are found in a sample of h2o(s)?
The covalent bonds could occur in a polyatomic ion in either the cation or the anion.
In a covalent bond, the atoms bond by sharing electrons. This 13 words question was answered by heather l. C)both covalent and ionic d)both covalent and metallic 3.the chemical bonding in sodium phosphate, na3po4, is classified as a)caco3 b)ch2cl2 c)ch3oh d)c6h12o6 4.which compound has both ionic and covalent bonding? Here calcium acts as the cation, with the carbonate species as the anion. Here calcium acts as the cation, with the carbonate species as the anion. When both ionic and covalent bonding occurs in a compound, the ionic portion is almost always between the cation and anion of the compound.
 Source: slideplayer.com
Source: slideplayer.com
Caf2 is an ionic compound because, in the caf2 molecule, calcium acts as a cation by donating its 2 extra electrons from the valence shell, and fluoride acts as an anion by accepting the electrons from calcium, completing its octet. Here calcium acts as the cation, with the carbonate species as the anion. The and ions in are connected with ionic bonds.
 Source: sciencenotes.org
Source: sciencenotes.org
Therefore, has both ionic and covalent bonds. The attraction between different atoms, molecules and ions is known as a chemical bond. Ionic bonds form between a metal and a nonmetal.
 Source: clutchprep.com
Source: clutchprep.com
If there is no link between the two ionic fragments, we should call the compound an ionic one (usually ‘salt’). Which compound contains both ionic and covalent bonds? Covalent bonds usually occur between nonmetals.
 Source: slideserve.com
Source: slideserve.com
Covalent bonds usually occur between nonmetals. Both ionic and hydrogen bonds d. The ammonium ion is polyatomic, which means it forms ionic salts.
 Source: thoughtco.com
Source: thoughtco.com
This 13 words question was answered by heather l. Ammonium chloride is an ionic compound. Which compound has both ionic and covalent bonding 1 caco3 2 ch2cl2 3 ch3oh 4 c6h12o6?
 Source: slideserve.com
Source: slideserve.com
Therefore, has both ionic and covalent bonds. Therefore, has both ionic and covalent bonds. We can distinguish between them a priori by asking ourselves whether there is a contiguous chain of mostly covalent bonds between each side of the ionic interaction.
 Source: slidetodoc.com
Source: slidetodoc.com
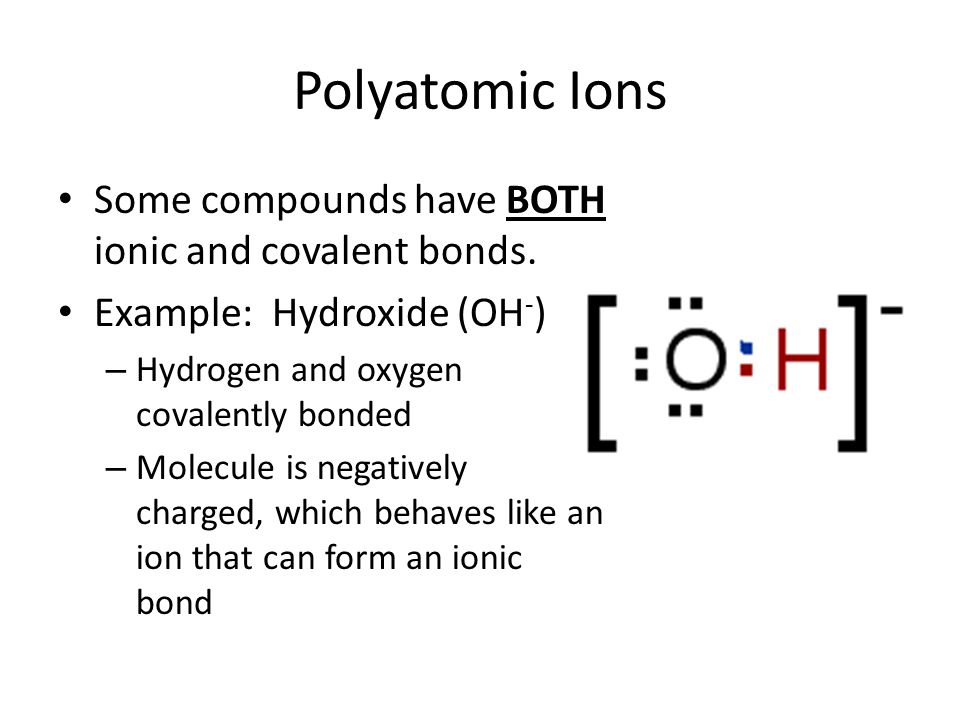
So na2co3 has both ionic and covalent bonds. Here, covalent bond is present between o and h forming ion. If there is no link between the two ionic fragments, we should call the compound an ionic one (usually ‘salt’).
 Source: brainly.in
Source: brainly.in
So, is caf2 ionic or covalent? If there is no link between the two ionic fragments, we should call the compound an ionic one (usually ‘salt’). So na2co3 has both ionic and covalent bonds.
 Source: doubtnut.com
Source: doubtnut.com
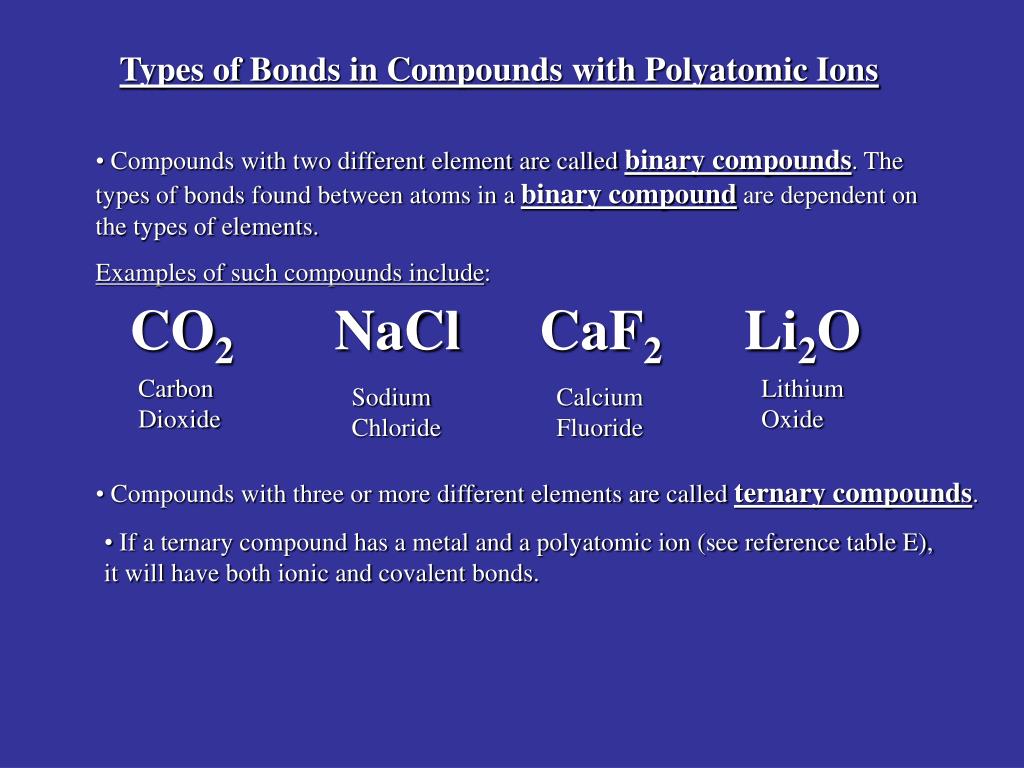
For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. Calcium carbonate is another example of a compound with both ionic and covalent bonds. Which compound contains both covalent and ionic bonds?
 Source: clutchprep.com
Source: clutchprep.com
Calcium carbonate is another example of a compound with both ionic and covalent bonds. In a covalent bond, the atoms bond by sharing electrons. These atoms (one atom and four atoms) are connected with covalent bonds.
 Source: toppr.com
Source: toppr.com
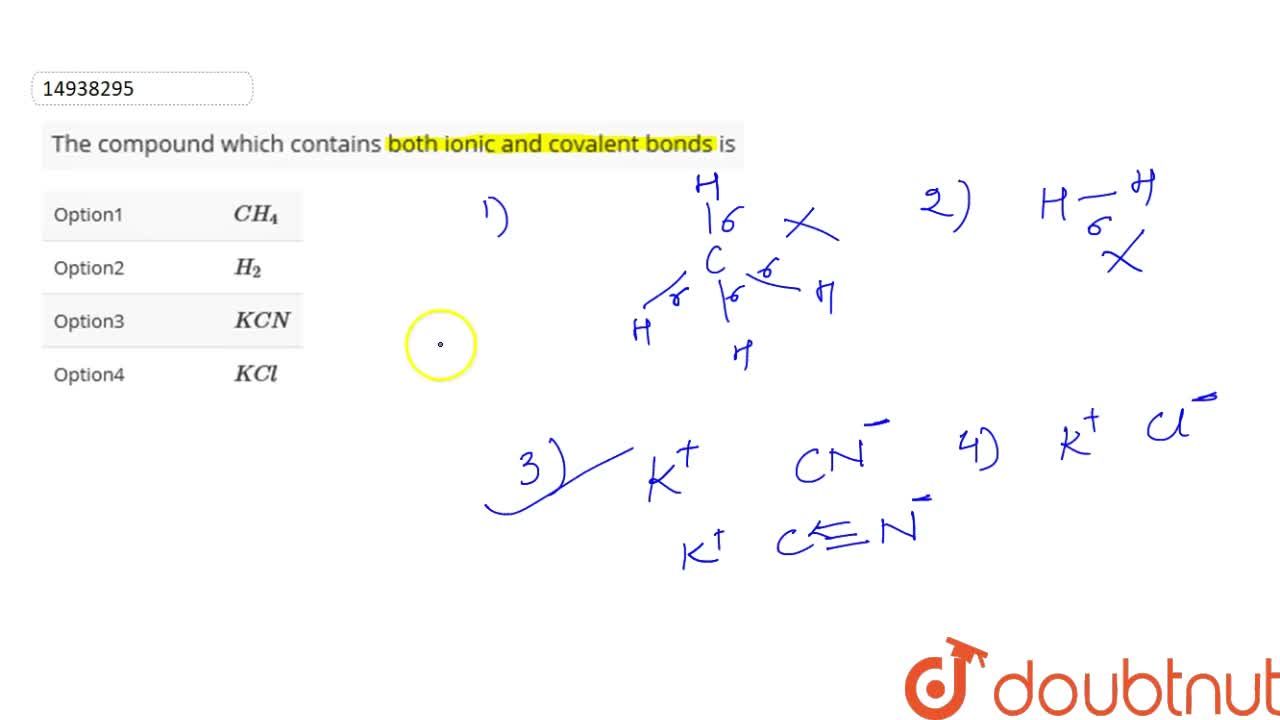
The covalent bonds could occur in a polyatomic ion in either the cation or the anion. Right here calcium acts because the cation, with the carbonate species because the anion. In kcn, the bonding between potassium ion and cyanide ion is ionic while carbon and nitrogen are covalently bonded in cyanide ion as:

When both ionic and covalent bonding occurs in a compound, the ionic portion is almost always between the cation and anion of the compound. A)the entropy of the atoms b)the atomic number of the atoms c)the first ionization energy of the atoms d)the polarity of the bond. Covalent bonds form between two nonmetals.
 Source: chegg.com
Source: chegg.com
Why is it true to say that calcium carbonate has both ionic and covalent bonds? Here, covalent bond is present between o and h forming ion. In a covalent bond, the atoms bond by sharing electrons.

Usually, there is some polarity (polar covalent bond) in which the electrons are shared, but spend more time with one atom than the other. These species share an ionic bond, while the carbon and oxygen atoms in carbonate are covalently bonded. Which kinds of bonds are found in a sample of h2o(s)?
 Source: slideplayer.com
Source: slideplayer.com
A) n h 4 c l contains both an iconic and covalent bond. Naphthalene, as a covalent compound, is made up of covalent molecules only. The and ions in are connected with ionic bonds.
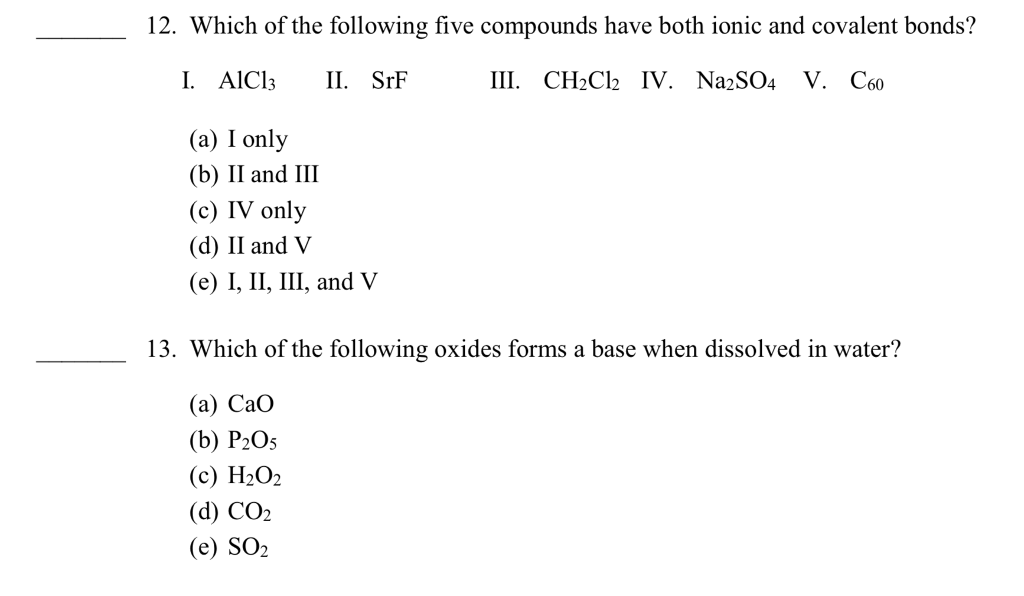 Source: chegg.com
Source: chegg.com
In other words, each ion contains more than one atoms. What make special is that its cation is polyatomic. Which compound contains both ionic and covalent bonds?
 Source: toppr.com
Source: toppr.com
Also know, does nacl contain both ionic and covalent bonds? These species share an ionic bond, while the carbon and oxygen atoms in carbonate are covalently bonded. In a covalent bond, the atoms bond by sharing electrons.
 Source: chegg.com
Source: chegg.com
Therefore, has both ionic and covalent bonds. These atoms (one atom and four atoms) are connected with covalent bonds. Here calcium acts as the cation, with the carbonate species as the anion.
 Source: intelwriters.com
Source: intelwriters.com
The compound that has both ionic and covalent bonding is. Comparison of ionic and covalent bonds ionic bonds usually occur between. Also know, does nacl contain both ionic and covalent bonds?
 Source: clutchprep.com
Source: clutchprep.com
Calcium carbonate is another example of a compound with both ionic and covalent bonds. To see more answers head over to college study guides. In other words, each ion contains more than one atoms.
Also Read :