0.04 m urea [ (nh_2)_2c = o] 0.01 m agno_3 0.03 m cuso_4 which aqueous solution has the highest freezing point? Al2(s o4)3 a l 2 ( s o 4) 3 provides five ions on ionisation as.
Which Aqueous Solution Will Have The Lowest Freezing Point. Depression in freezing point is a colligative property which depends upon the amount of the solute. If the molecules mass of �a�is 60, then the molecules mass of �b� will be: Which aqueous solution will have the lowest freezing point? Osmolality = i × m.
 Solved ) Which Of The Following Aqueous Solutions Will Have | Chegg.com From chegg.com
Solved ) Which Of The Following Aqueous Solutions Will Have | Chegg.com From chegg.com
Related Post Solved ) Which Of The Following Aqueous Solutions Will Have | Chegg.com :
Thus 1% c a c l 2 produces most ions and thus lowers the freezing point to the maximum value. I= 1 as it is a non electrolyte and does not dissociate. While ki provides two ions and c 5h 10o5 c 5 h 10 o 5 and c 12h 22o11 c 12 h 22 o 11 are not ionised, so they have single particle. Osmolality = i × m.
Please log in or register to add a comment.
Freezing point of a pure solvent depends on. Of particles of solute.but freezing point &boiling point are not colligative properties.so in this question lowest freezing point is of 0.1 m al 2 (so 4) 3 due to greater no. Which of the following aqueous solutions has the lowest freezing point? Thus, δ t f ( max) is for 0.10 m alluminium. 0.1mcai2 will have the lowest freezing point, followed by 0.1mnacl, and the highest of the three solutions will be 0.1mc6h12o6, but all three of them will have a. 0.1mcai2 will have the lowest freezing point, followed by 0.1mnacl, and the highest of the three solutions will be 0.1mc6h12o6, but all three of them will have a lower freezing point than pure water.
 Source: slideserve.com
Source: slideserve.com
A) al2 (so4)3 b) c6h12o6 c) ki d) c12h22o11. Change in freezing point from pure solvent (water) to solution (sodium chloride solution): If the molecules mass of �a�is 60, then the molecules mass of �b� will be:
 Source: doubtnut.com
Source: doubtnut.com
Which of the following 0.1 m aqueous solution will have the lowest freezing point? The highest freezing point will be of the solution having the lowest δt f. Which aqueous solution will have the lowest freezing point?
 Source: doubtnut.com
Source: doubtnut.com
Hence, the correct option is, 2.0 m nacl. 0.40 m c 2 h 6 o 2 Freezing point depression is a function of the amount of dissolved substance — but substance must be treated as distinct particles rather than compound.
 Source: chegg.com
Source: chegg.com
Which of the following 0.1 m aqueous solution will have the lowest freezing point? Which of the following 0.1 m aqueous solution will have the lowest freezing point? Depression in freezing point is a colligative property which depends upon the amount of the solute.
 Source: chegg.com
Source: chegg.com
Which of the following 0.1 m aqueous solution will have the lowest freezing point ? 0.1mcai2 will have the lowest freezing point, followed by 0.1mnacl, and the highest of the three solutions will be 0.1mc6h12o6, but all three of them will have a lower freezing point than pure water. Sodium chloride gives two ions and potassium sulphate gives three ions per formula unit.

Which of the following 0.1 m aqueous solutions will have the lowest freezing point? Which of the following aqueous solutions has the lowest freezing point? 0.04 m urea [ (nh_2)_2c = o] 0.01 m agno_3 0.03 m cuso_4 which aqueous solution has the highest freezing point?
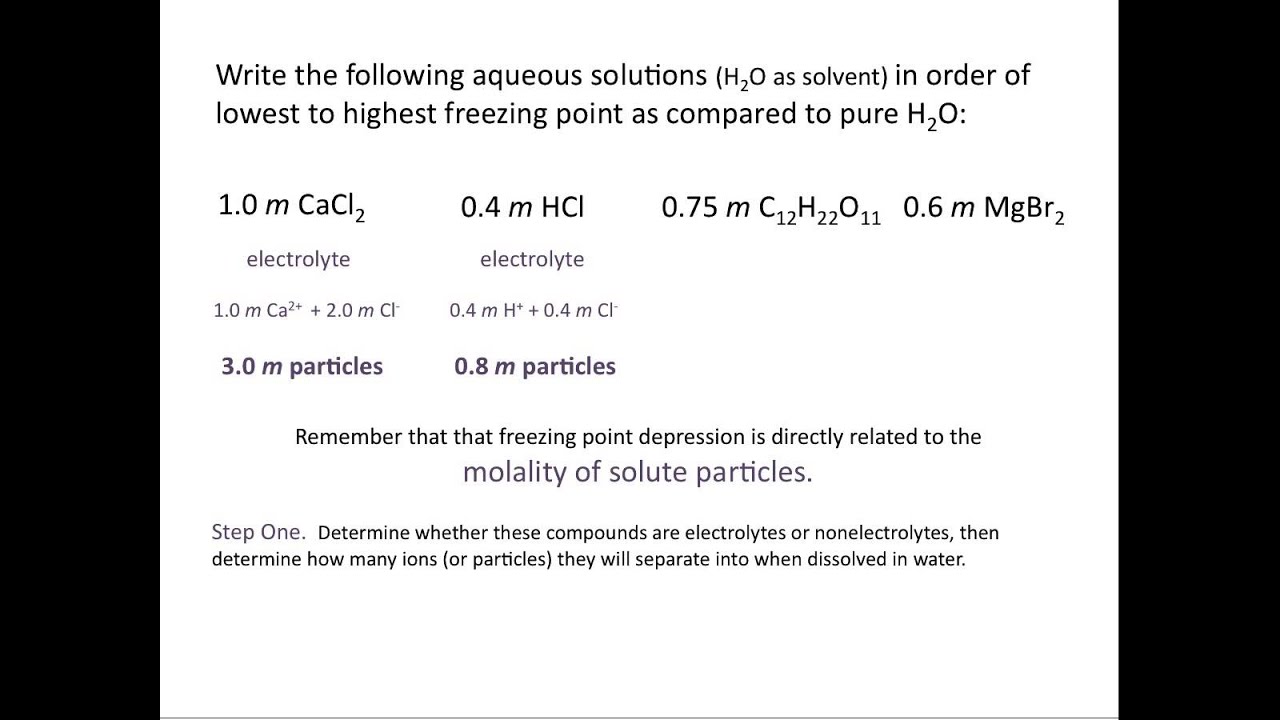 Source: youtube.com
Source: youtube.com
So, most particles of the solute is going to be in the solution of nacl, so the lowest freezing point has the aqueous solution of nacl. So, most particles of the solute is going to be in the solution of nacl, so the lowest freezing point has the aqueous solution of nacl. Of particles of solute.but freezing point &boiling point are not colligative properties.so in this question lowest freezing point is of 0.1 m al 2 (so 4) 3 due to greater no.
 Source: chegg.com
Source: chegg.com
Which of the following 0.10 m aqueous solutions will have the lowest freezing point ? (a) 0.2 m nacl (b) 0.2 m cacl2 (c) 0.2 m h2so4 (d) 0.2 m nh3 (e) 0.2 m al(no3)3 answer and explanation: Freezing point depression is a function of the amount of dissolved substance — but substance must be treated as distinct particles rather than compound.
 Source: bartleby.com
Source: bartleby.com
Which aqueous solution has the lowest freezing point? This is an important distinction because ionic compounds, when they dissolve in water, typically separate into hydrated anions and hydrated cations — two distinct species as far as freezing point depression is concerned. Remember, the greater the concentration of particles, the lower the freezing point will be.
 Source: chegg.com
Source: chegg.com
(a) 0.040 m glucose (b) 0.025 m kbr (c) 0.020 m nacl (d) 0.020 m. Which of the following aqueous solutions has the lowest freezing point? (a) k2so4 (b) na3po4 (c) cacl2 (d) nacl 17.
 Source: chegg.com
Source: chegg.com
Which of the following 0.1 m aqueous solution will have highest boiling point? Depression in freezing point is a colligative property which depends upon the amount of the solute. Which aqueous solution will have the lowest freezing point?
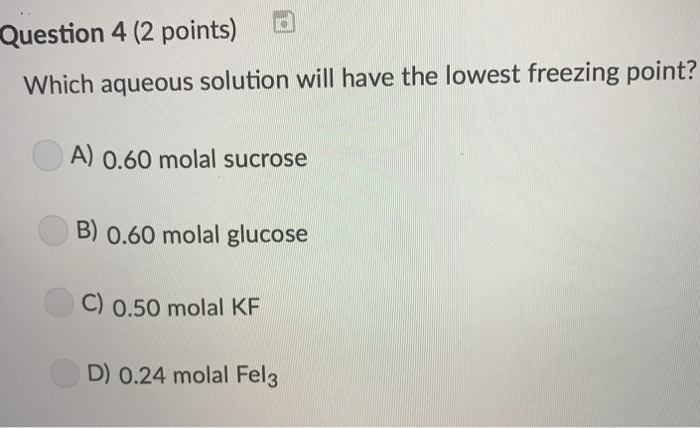
From the given options, 2.0 m nacl has the lowest freezing point. Of particles & hence greater deppression in freezing point takes place.and in case of boiling point. Which would have the lowest freezing point?
 Source: chegg.com
Source: chegg.com
0.1mcai2 will have the lowest freezing point, followed by 0.1mnacl, and the highest of the three solutions will be 0.1mc6h12o6, but all three of them will have a. Freezing point of a pure solvent depends on. Which would have the lowest freezing point?
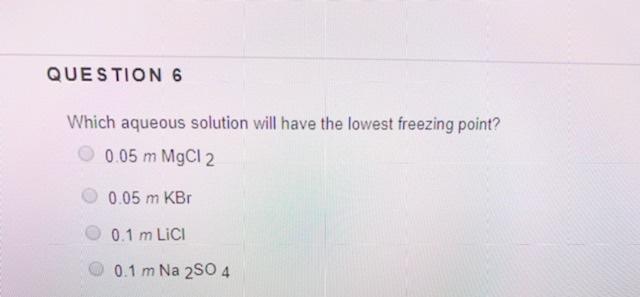
A 0.1 m aqueous solution is made of each of the substances listed. Thus as molarity and boiling point constant is same for all electrolytes, when i=4, elevation in boiling point will be highest. Also, 1% nacl will have van�t hoff factor 2 and 1 % c a c l 2 will have vant hoff factor i as 3.
 Source: chegg.com
Source: chegg.com
Change in freezing point from pure solvent (water) to solution (sodium chloride solution): Which aqueous solution will have the lowest freezing point? 0.5 l of the same solution on treatment with excess of a g n o 3 solutionwill yield (assume a = 1):
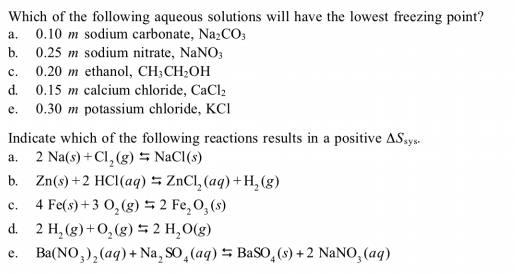 Source: bartleby.com
Source: bartleby.com
Which aqueous solution will have the lowest freezing point? This is an important distinction because ionic compounds, when they dissolve in water, typically separate into hydrated anions and hydrated cations — two distinct species as far as freezing point depression is concerned. Which aqueous solution has lowest freezing point?
 Source: chegg.com
Source: chegg.com
0.1mcai2 will have the lowest freezing point, followed by 0.1mnacl, and the highest of the three solutions will be 0.1mc6h12o6, but all three of them will have a lower freezing point than pure water. Of particles & hence greater deppression in freezing point takes place.and in case of boiling point. A 0.1 m aqueous solution is made of each of the substances listed.
 Source: youtube.com
Source: youtube.com
Thus, δ t f ( max) is for 0.10 m alluminium. 0.04 m urea [ (nh_2)_2c = o] 0.01 m agno_3 0.03 m cuso_4 which aqueous solution has the highest freezing point? Which of the following 0.1 m aqueous solution will have the lowest freezing point ?
![Solved] Which Aqueous Solution Will Have The Lowest Freezing Point? | Course Hero](https://www.coursehero.com/qa/attachment/11601488/ “Solved] Which Aqueous Solution Will Have The Lowest Freezing Point? | Course Hero”) Source: coursehero.com
Sodium chloride gives two ions and potassium sulphate gives three ions per formula unit. 0.04 m urea [ (nh_2)_2c = o] 0.01 m agno_3 0.03 m cuso_4 which aqueous solution has the highest freezing point? Remember, the greater the concentration of particles, the lower the freezing point will be.

Which of the following 0.1 m aqueous solution will have the lowest freezing point? Δt = i · kf · c. (a) 0.2 m nacl (b) 0.2 m cacl2 (c) 0.2 m h2so4 (d) 0.2 m nh3 (e) 0.2 m al(no3)3 answer and explanation:
Also Read :





