Specifically, the means by which circularly polarized light could create chiral amino acids, both on earth and in outer space, are detailed. Let's look at chirality in proteins and dna.
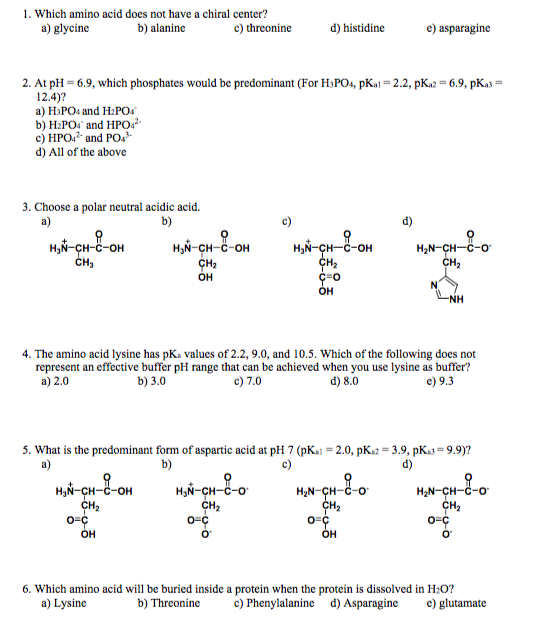
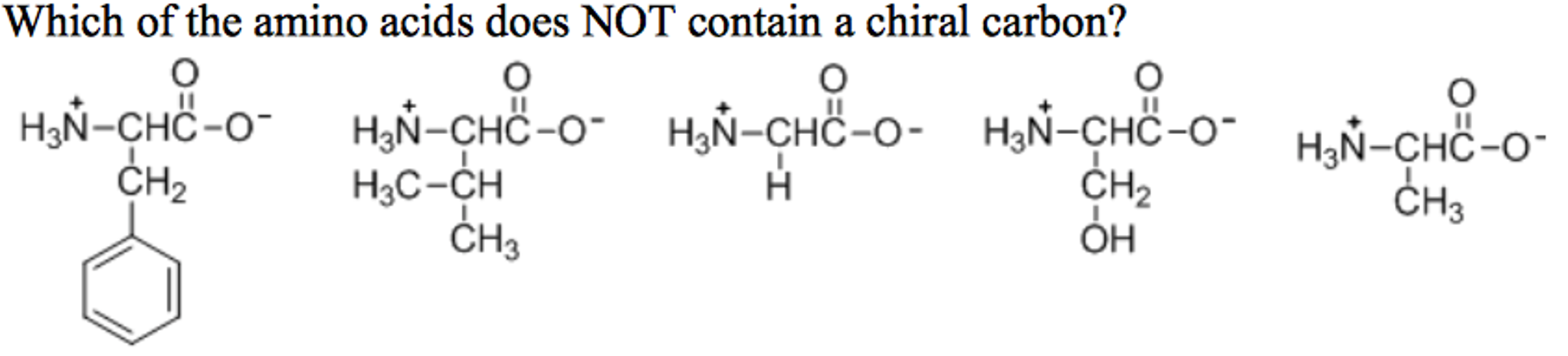
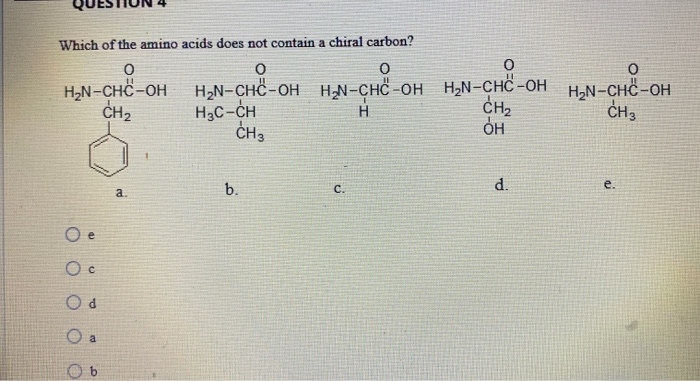
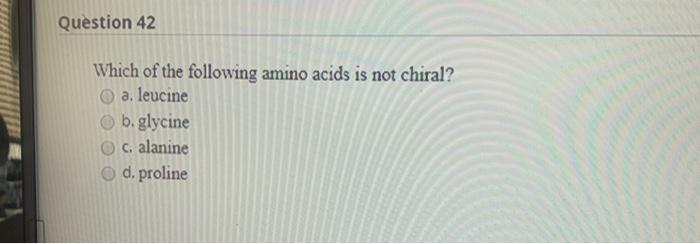
Which Amino Acid Is Not Chiral. Glycine is not chiral because it has 2 hydrogens attached to its central alpha carbon. All amino acids except glycine are chiral because they all contain at least one chiral centre. Objects are chiral when they cannot be superposed on their mirror images. Chapter 27—amino acids and proteins multiple choice 1.
 D-And L-Configurations Of A Chiral Amino Acid. R Is The Side Chain Of… | Download Scientific Diagram From researchgate.net
D-And L-Configurations Of A Chiral Amino Acid. R Is The Side Chain Of… | Download Scientific Diagram From researchgate.net
Related Post D-And L-Configurations Of A Chiral Amino Acid. R Is The Side Chain Of… | Download Scientific Diagram :
With the expansion of the application of chiral aldehyde. Why are all amino acids chiral? In the past they were often called imino acids, a misnomer because they do not contain an imine grouping hn=c. These include proline and hydroxyproline, which are secondary amines.
L amino acids are a subcategory of amino acids.
For example, the left and right hands of a human are approximately mirror images of each other but are not their own mirror images, so they are chiral. Racemic mixtures (or solutions) of molecules do not exhibit optical activity because there are The obsolete term remains frequent. Very hydrophobicr group is an idole ring compressed of a benzene ring fused to pyrrole group. For example, the left and right hands of a human are approximately mirror images of each other but are not their own mirror images, so they are chiral. As noted above, since r is just a hydrogen, glycine is the only natural amino acid that is not chiral at the alpha carbon.
 Source: chegg.com
Source: chegg.com
100% (1 rating) previous question next question. Objects are chiral when they cannot be superposed on their mirror images. L amino acids are a subcategory of amino acids.
 Source: chegg.com
Source: chegg.com
Specifically, the means by which circularly polarized light could create chiral amino acids, both on earth and in outer space, are detailed. Nature uses almost exclusively l. The general formula for an amino acid is.
 Source: chegg.com
Source: chegg.com
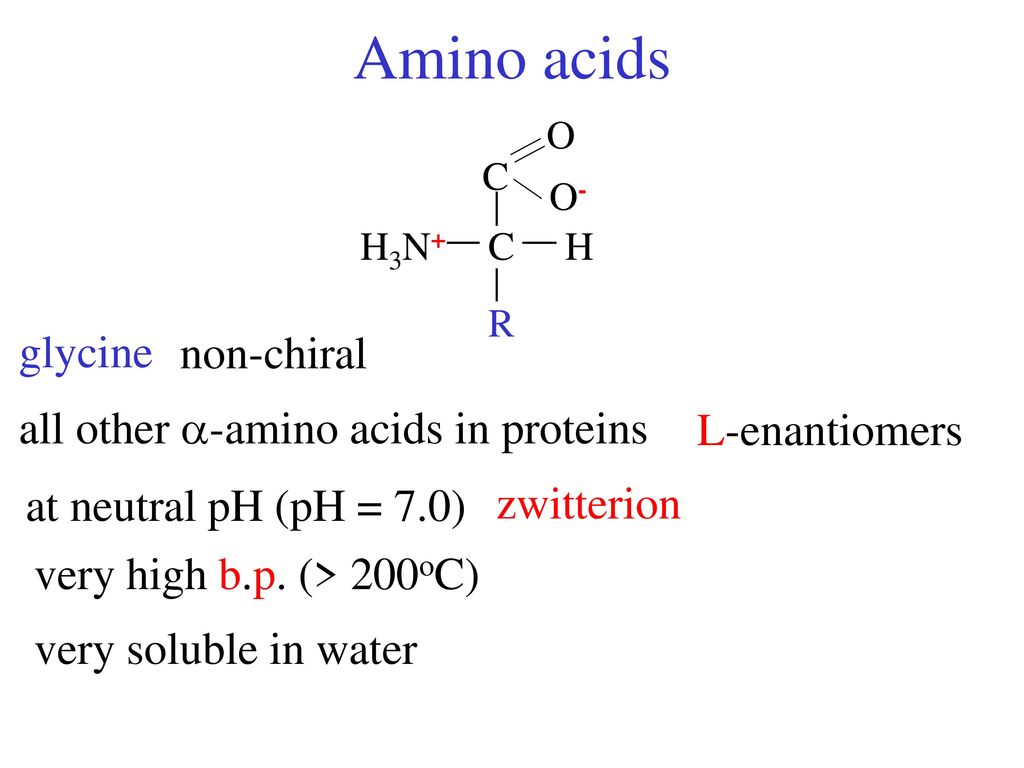
Chapter 27—amino acids and proteins multiple choice 1. In the case of the amino acid, this includes the carboxylic acid functional group, the amine functional group, the various r‐groups, and the hydrogen. With the exception of glycine, which is not chiral, these amino acids have an s configuration and are designated as l using the fischer configurational system.
 Source: thoughtco.com
Source: thoughtco.com
And, finally, the possibility that chiral selection might have occurred on solid surfaces is mentioned. Which of the following amino acids is not chiral? The central carbon has four different groups attached.
 Source: chegg.com
Source: chegg.com
The designed fluorescent probes consist of three components: Although in some classification schemes, glycine is considered nonpolar, hydrogen is so small that it contributes negligibly to nonpolar surface area. In the past they were often called imino acids, a misnomer because they do not contain an imine grouping hn=c.
 Source: alevelchemistry.co.uk
Source: alevelchemistry.co.uk
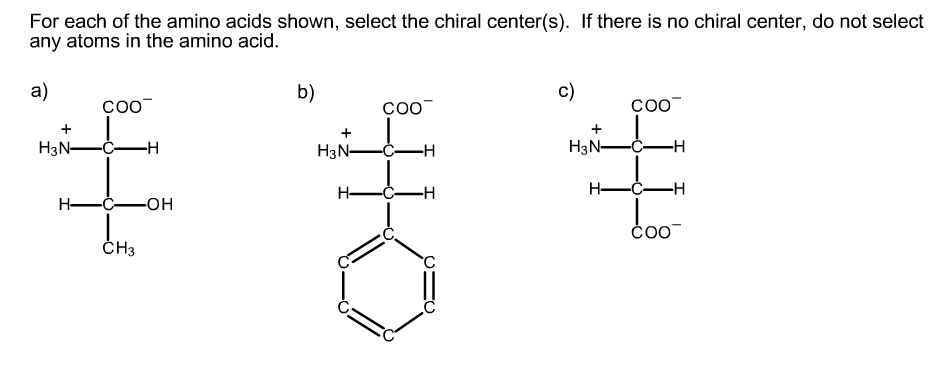
Cysteine is (r) and glycine is not chiral. All amino acids except glycine are chiral because they all contain at least one chiral centre. Which amino acid is not chiral?
 Source: chegg.com
Source: chegg.com
Experts are tested by chegg as specialists in their subject area. Although in some classification schemes, glycine is considered nonpolar, hydrogen is so small that it contributes negligibly to nonpolar surface area. Next, several models that rely on the weak interaction to select chirality are described.
 Source: chegg.com
Source: chegg.com
Although in some classification schemes, glycine is considered nonpolar, hydrogen is so small that it contributes negligibly to nonpolar surface area. As noted above, since r is just a hydrogen, glycine is the only natural amino acid that is not chiral at the alpha carbon. A leucine b isoleucine c glycine d alanine e threonine.
 Source: slideplayer.com
Source: slideplayer.com
So the compound can exist as a pair of nonsuperimposable mirror images. For example, the left and right hands of a human are approximately mirror images of each other but are not their own mirror images, so they are chiral. Although in some classification schemes, glycine is considered nonpolar, hydrogen is so small that it contributes negligibly to nonpolar surface area.

The fact that chirality was missing in those amino acids is not just a problem to be debated, it points to a catastrophic failure that life cannot come from chemicals by natural processes. The designed fluorescent probes consist of three components: These include proline and hydroxyproline, which are secondary amines.
 Source: youtube.com
Source: youtube.com
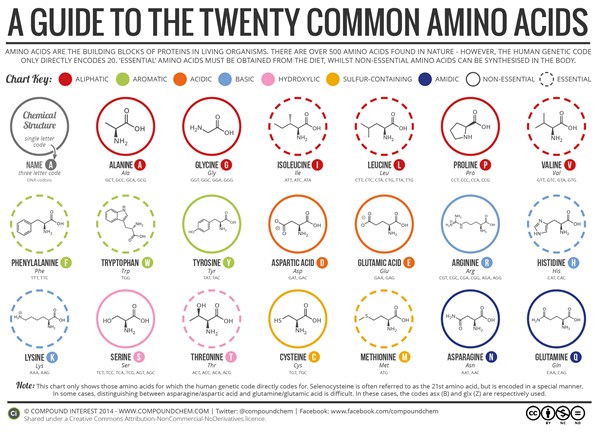
So the compound can exist as a pair of nonsuperimposable mirror images. They come in l or d. Stereochemistry of amino acids other terms you may encounter:

They come in l or d. Very hydrophobicr group is an idole ring compressed of a benzene ring fused to pyrrole group. In the past they were often called imino acids, a misnomer because they do not contain an imine grouping hn=c.
Why are all amino acids chiral? The obsolete term remains frequent. Chiral recognition and quantification of amino acid (aa) enantiomers are of great significance in many fields such as biology and biomedicine.
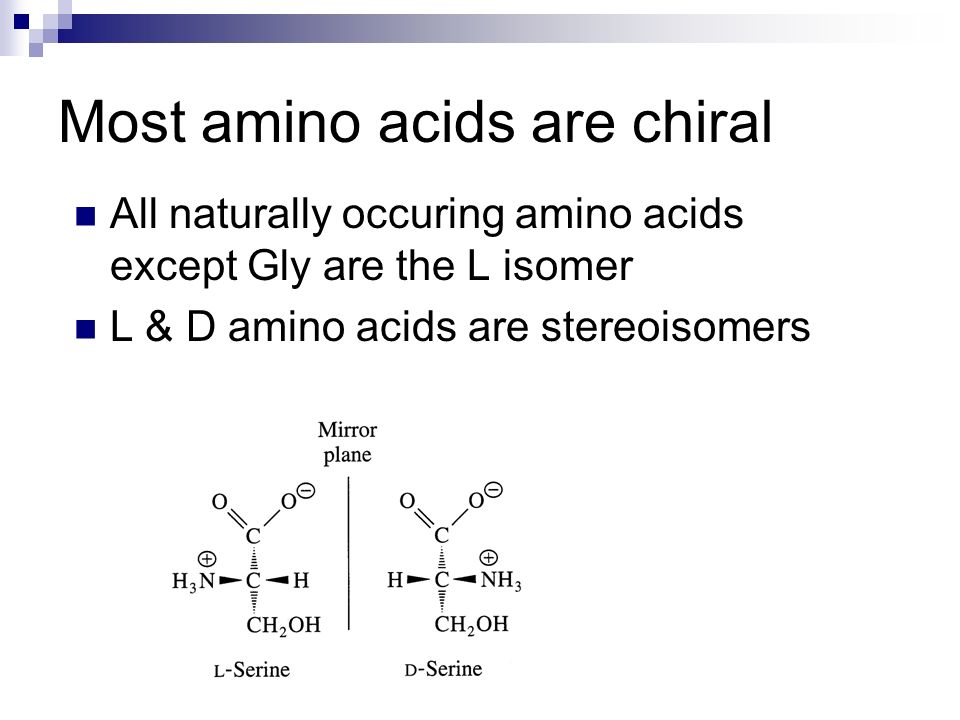 Source: slideplayer.com
Source: slideplayer.com
Regarding the (s) and (r) nomenclature, nearly all amino acids in proteins are (s) at the alpha carbon. Some amino acids are chiral. For example, the left and right hands of a human are approximately mirror images of each other but are not their own mirror images, so they are chiral.
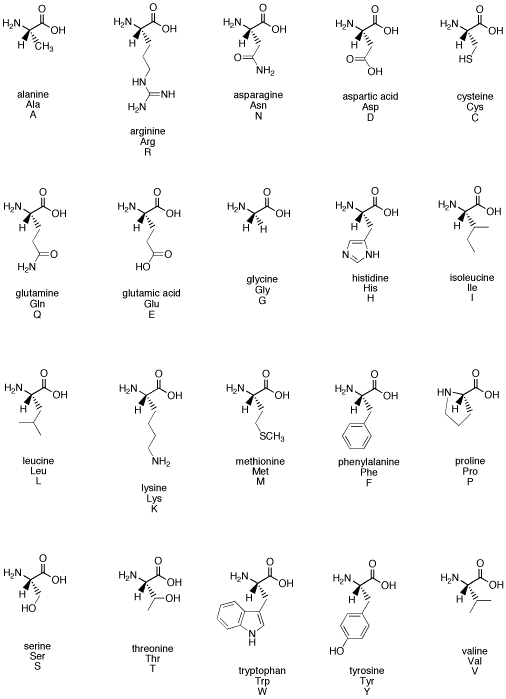 Source: employees.csbsju.edu
Source: employees.csbsju.edu
Nature uses almost exclusively l. Homochirality is a uniformity of chirality, or handedness. For example, the left and right hands of a human are approximately mirror images of each other but are not their own mirror images, so they are chiral.
 Source: thoughtco.com
Source: thoughtco.com
As noted above, since r is just a hydrogen, glycine is the only natural amino acid that is not chiral at the alpha carbon. Amino acids are chiral (except for glycine) because they have 4 different constituents or groups connected to a central alpha carbon. The simplest amino acid is glycine, where the r is an h nh2 ch2 co2h some amino acids have an extra carboxylic acid or an amine group on the r group.
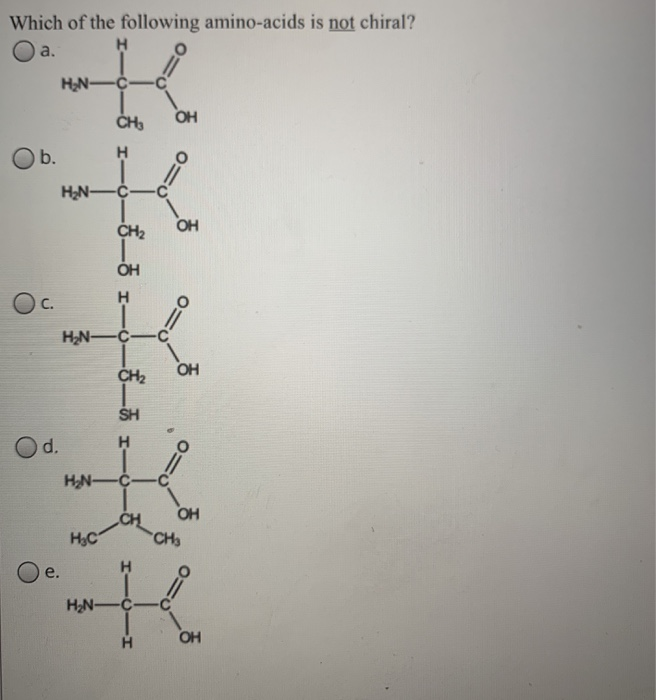 Source: chegg.com
Source: chegg.com
They come in l or d. Objects are chiral when they cannot be superposed on their mirror images. With the exception of glycine, which is not chiral, these amino acids have an s configuration and are designated as l using the fischer configurational system.
 Source: slideplayer.com
Source: slideplayer.com
Regarding the (s) and (r) nomenclature, nearly all amino acids in proteins are (s) at the alpha carbon. Why are all amino acids chiral? Racemic mixtures (or solutions) of molecules do not exhibit optical activity because there are
 Source: researchgate.net
Source: researchgate.net
L amino acids are a subcategory of amino acids. Regarding the (s) and (r) nomenclature, nearly all amino acids in proteins are (s) at the alpha carbon. Chiral recognition and quantification of amino acid (aa) enantiomers are of great significance in many fields such as biology and biomedicine.
 Source: nature.com
Source: nature.com
Very hydrophobicr group is an idole ring compressed of a benzene ring fused to pyrrole group. Objects are chiral when they cannot be superposed on their mirror images. The central carbon has four different groups attached.
Also Read :





