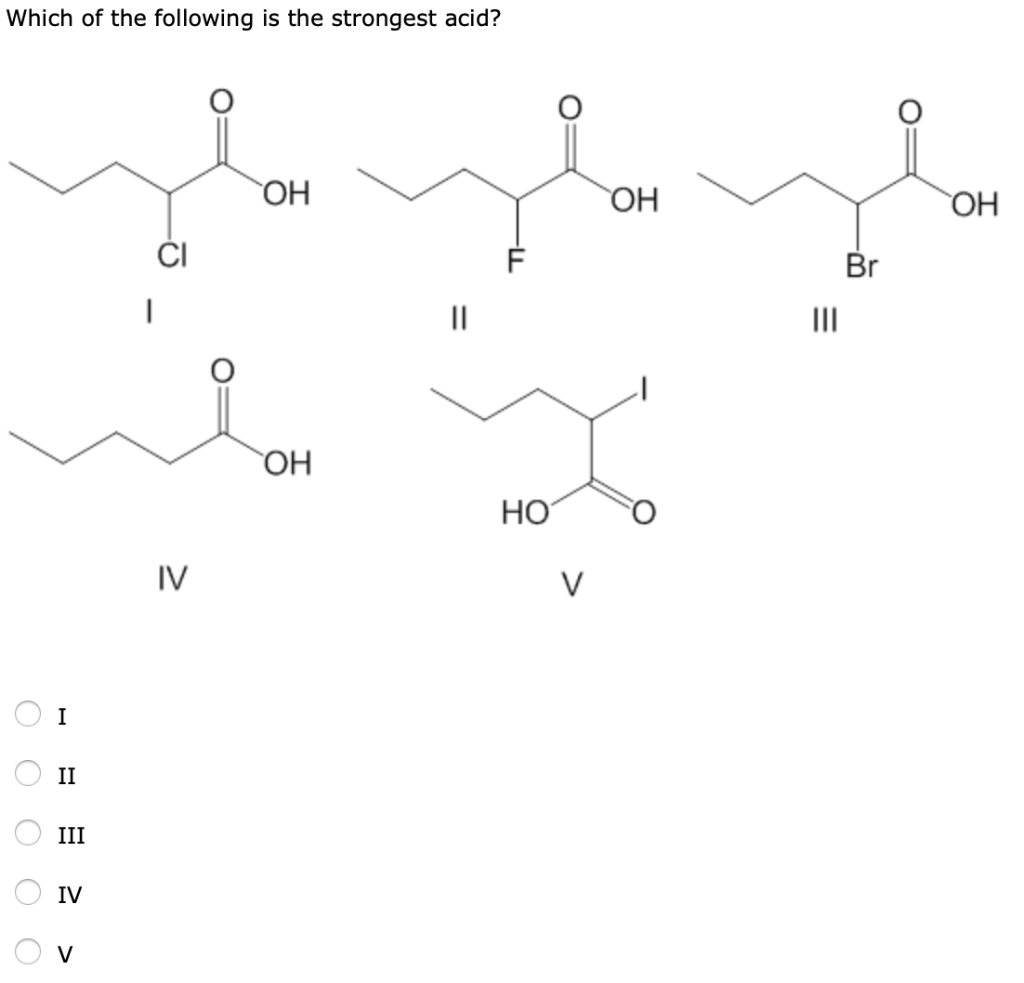
So that means that this one is going to be the strongest asset because he form this aromatic ring in our contact base. In aqueous solutions it ionizes completely to form hydronium ions (h 3 o +) and hydrogen sulfate ions (hso 4 −).
Of The Following Which Is The Strongest Acid. C 6h 5c ooh (pk a = 4.22) c 6 h 5 c o o h ( p k a = 4.22) c. These acids are so strong that they aren’t even considered conventional acids; Among the given acids, perchloric acid h c l o 4 is the strongest acid. 9) which of the following is the strongest acid?
 Solved Which Of The Following Is The Strongest Acid? Тон Ho | Chegg.com From chegg.com
Solved Which Of The Following Is The Strongest Acid? Тон Ho | Chegg.com From chegg.com
Related Post Solved Which Of The Following Is The Strongest Acid? Тон Ho | Chegg.com :
Chloric acid, hydrobromic acid, hydrochloric acid, hydroiodic acid, nitric acid, perchloric acid, and sulfuric. A) hydroiodic acid, hi b) hydrochloric acid, hcl c) carbonic acid, h 2 co d) nitric acid, hno e) perchloric acid, hcl 3 3. Among the following compounds, the strongest acid is; The strongest acid is hclo4.
In aqueous solutions it ionizes completely to form hydronium ions (h 3 o +) and hydrogen sulfate ions (hso 4 −).
C h 2c lc ooh c h 2 c l c o o h. A) hydroiodic acid, hi b) hydrochloric acid, hcl c) carbonic acid, h 2 co d) nitric acid, hno e) perchloric acid, hcl 3 3. The second and third protons are split off the acid molecule even less. Sulfuric acid is a very strong acid; There are 7 solid acids: This substance is so strong it will eat through skin, bones, and pretty much any container used to.
 Source: bartleby.com
Source: bartleby.com
What is the ph of the solution? Among the following the strongest acid is. Chloric acid (denoted by the chemical formula hclo3) perchloric acid (denoted by the chemical formula hclo4) these acids are classified as strong acids because they (almost) completely dissociate into their constituent ions, which includes their anionic conjugate base and a proton, when they are dissolved in water.
 Source: chegg.com
Source: chegg.com
Fluoroantimonic acid is the world’s strongest acid, proudly standing on the pedestal slightly above carborane. The second and third protons are split off the acid molecule even less. The stability of the conjugate base decides the strength of the acid.
 Source: youtube.com
Source: youtube.com
The pressure in the container following evaporation is 892 torr. Chloric acid, hydrobromic acid, hydrochloric acid, hydroiodic acid, nitric acid, perchloric acid, and sulfuric. The stability of the conjugate base decides the strength of the acid.
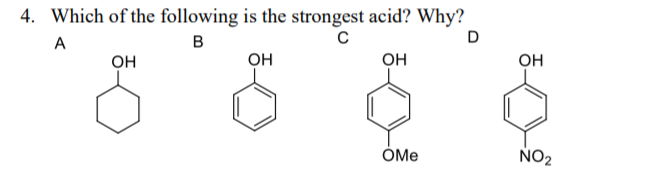
Closed dec 19, 2021 by chithrabasu. Larger the k a (dissociation constant) of an acid, more will be the h + ions produced, hence, stronger will be the acid. The acid is followed by its ka value.
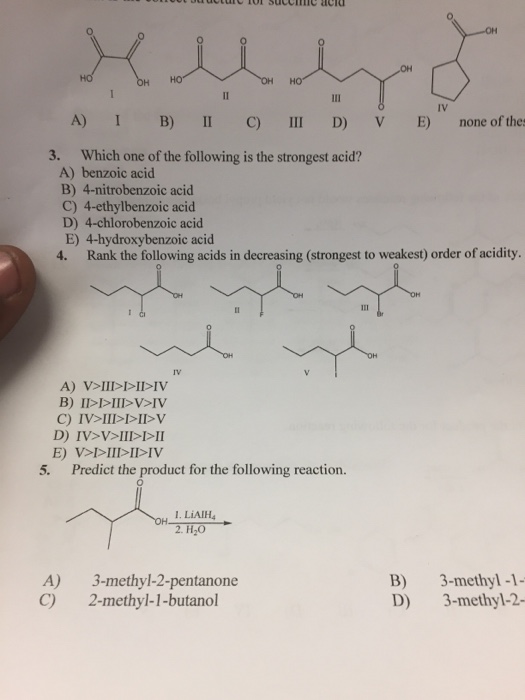
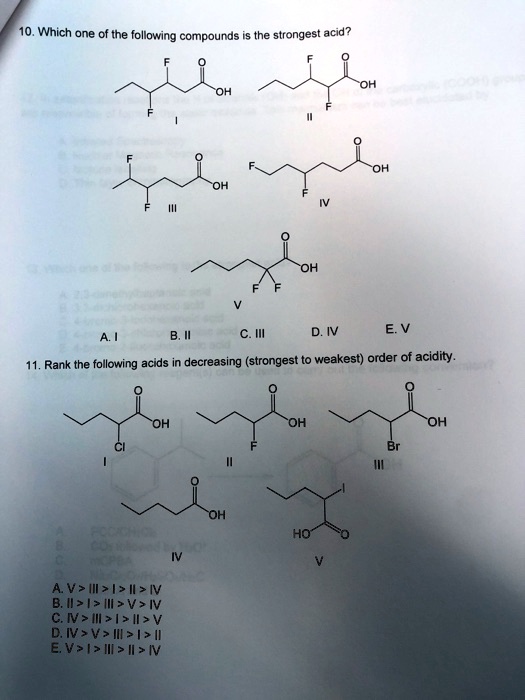 Source: numerade.com
Source: numerade.com
H c ooh (pk a = 3.77) h c o o h ( p k a = 3.77) b. Compared to all the given choices, perchloric acid shows the highest oxidation number on chlorine or. Now to determine which acid is the strongest.
 Source: oneclass.com
Source: oneclass.com
So, like i said before, the one with the most stable conjugated base is going to be the strongest asset because it�s easiest to form that face because it�s the most stable. So this one is non aromatic in its residents contributors. C h 3c ooh c h 3 c o o h.
C 6h 5c ooh (pk a = 4.22) c 6 h 5 c o o h ( p k a = 4.22) c. D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. Sulfuric acid is a very strong acid;
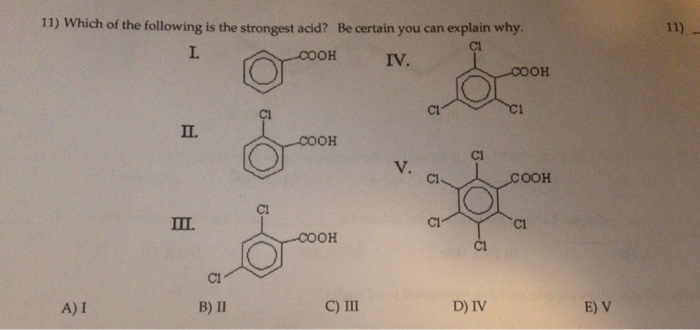
![Solved] 17. Which Of The Following Is The Strongest Acid? A. Hf B. Hbr C. Hci D. Hi 18. Which Of The Following Is The Strongest… | Course Hero](https://www.coursehero.com/qa/attachment/14950263/ “Solved] 17. Which Of The Following Is The Strongest Acid? A. Hf B. Hbr C. Hci D. Hi 18. Which Of The Following Is The Strongest… | Course Hero”) Source: coursehero.com
- which of the following is the strongest acid? 9) which of the following is the strongest acid? H c ooh (pk a = 3.77) h c o o h ( p k a = 3.77) b.
 Source: youtube.com
Source: youtube.com
Closed dec 19, 2021 by chithrabasu. The stability of the conjugate base decides the strength of the acid. There are 7 strong acids:
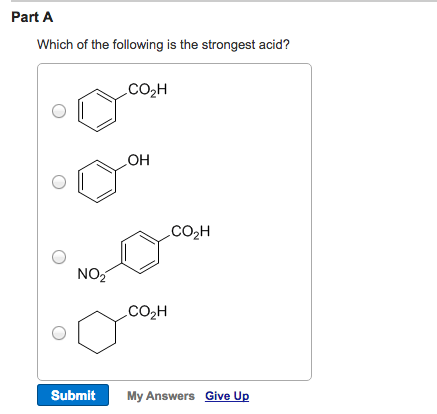 Source: chegg.com
Source: chegg.com
C h 2c lc h 2c ooh c h 2 c l c h 2 c o o h. So this one is non aromatic in its residents contributors. There are 7 solid acids:
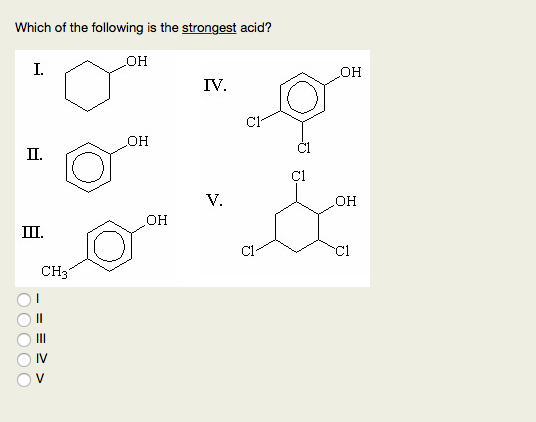 Source: chegg.com
Source: chegg.com
Perchloric acid is the strongest acid with molecular formula, $cl{o_3}(oh)$. Hence, we can understand that option d) is the correct answer for the given question. In aqueous solutions it ionizes completely to form hydronium ions (h 3 o +) and hydrogen sulfate ions (hso 4 −).
 Source: oneclass.com
Source: oneclass.com
This is because the conjugate base c l o 4 − ion is the weakest base. C 6 h 5 c o o h is the strongest acid. There are 7 strong acids:
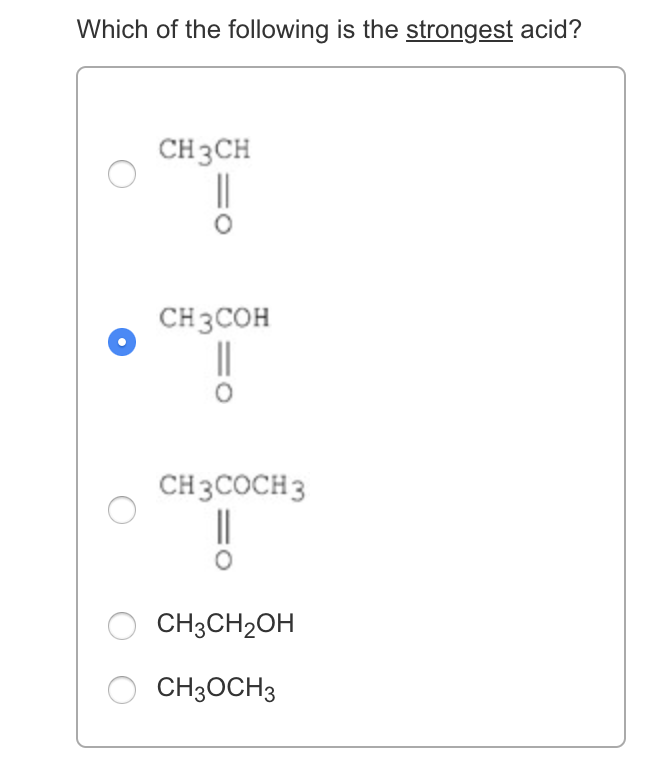 Source: chegg.com
Source: chegg.com
In alkali metal group which is the strongest reducing agent and why? Which of the following is strongest lewis acid; Closed dec 19, 2021 by chithrabasu.
 Source: youtube.com
Source: youtube.com
Closed dec 19, 2021 by chithrabasu. Perchloric acid is the strongest acid with molecular formula, $cl{o_3}(oh)$. A multiprotic acid�s ka decreases as it loses an h+.
 Source: toppr.com
Source: toppr.com
This substance is so strong it will eat through skin, bones, and pretty much any container used to. C h 2c lc ooh c h 2 c l c o o h. So this one is non aromatic in its residents contributors.
 Source: brainly.in
Source: brainly.in
The strongest acid is hclo4. Chloric acid (denoted by the chemical formula hclo3) perchloric acid (denoted by the chemical formula hclo4) these acids are classified as strong acids because they (almost) completely dissociate into their constituent ions, which includes their anionic conjugate base and a proton, when they are dissolved in water. So this one is non aromatic in its residents contributors.
 Source: clutchprep.com
Source: clutchprep.com
D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. A) hydroiodic acid, hi b) hydrochloric acid, hcl c) carbonic acid, h 2 co d) nitric acid, hno e) perchloric acid, hcl 3 3. What is the ph of the solution?
 Source: transtutors.com
Source: transtutors.com
This substance is so strong it will eat through skin, bones, and pretty much any container used to. Among the following compounds, the strongest acid is; C h 2c lc ooh c h 2 c l c o o h.
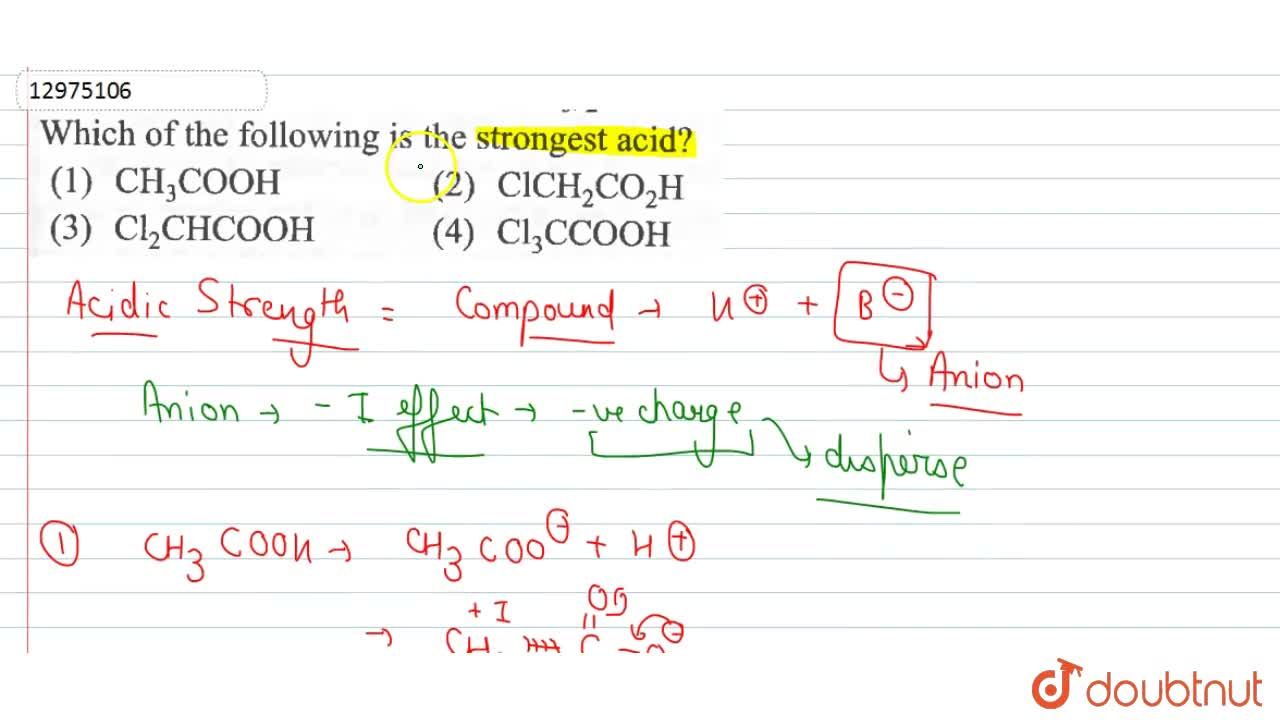 Source: doubtnut.com
Source: doubtnut.com
Which of the following is strongest lewis acid; Therefore, hocl is the the strongest acid and hoi is weakest, and also acid stamin decreases as the central halogen descends on the periodic table. Please log in or register to add a comment.
 Source: toppr.com
Source: toppr.com
Of the following, which is the strongest acid? Chloric acid, hydrobromic acid, hydrochloric acid, hydroiodic acid, nitric acid, perchloric acid, and sulfuric. H c ooh (pk a = 3.77) h c o o h ( p k a = 3.77) b.
Also Read :