Which of the following atoms has the smallest radius? This atomic mass represents the;
Of The Following Which Atom Has The Smallest Atomic Radius. A) down a group and from right to left across a period b) up a group and from left to right across a period c) down a group and from left to right across a period d) up a group and from right to left across a period e) down a group; Of the following, which atom has the smallest atomic radius? This homework question can be solved by thinking about how the charges react to each other. The period position has no effect
 Which Of These Atoms Has The Smallest Radi… | Clutch Prep From clutchprep.com
Which Of These Atoms Has The Smallest Radi… | Clutch Prep From clutchprep.com
Related Post Which Of These Atoms Has The Smallest Radi… | Clutch Prep :
A) na b) ba c) ca d) cs. Which of the transition metals has the smallest atomic radius; Thus, helium is the smallest element, and francium is the largest. An atom has a mass number of 32 and 17 neutrons.
Which has largest radius co 3?
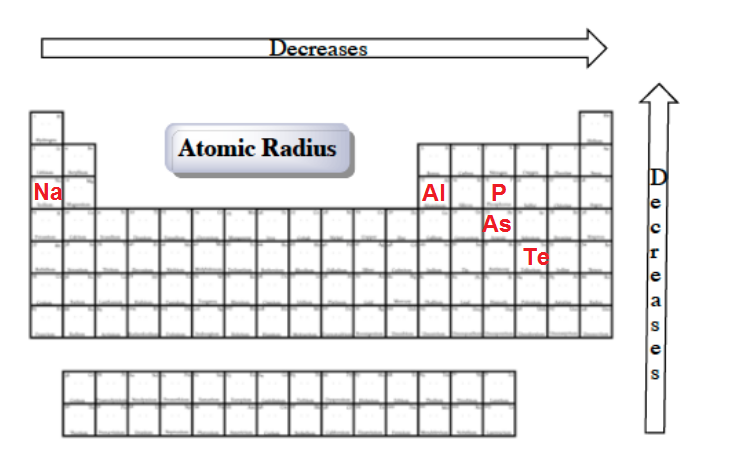
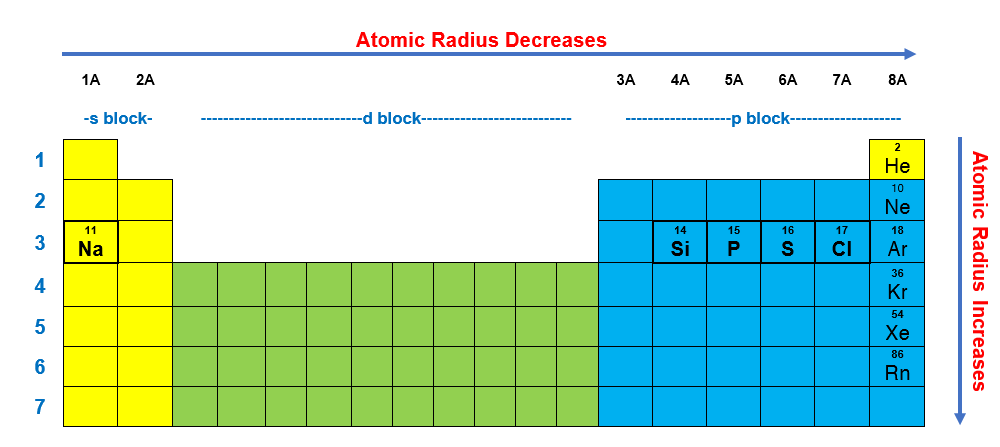
Which element has the bigger radius? Which atom has the highest first ionization energy? The concentration of more protons in the nucleus creates a higher effective nuclear charge, in other words, there is a stronger force of attraction pulling the electrons closer to the nucleus resulting in a smaller atomic radius. Since, the element which will in the right most of the theriodic table will be having smallest alomic radius. Of the following, which atom has the smallest atomic radius? Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top.
 Source: quizlet.com
Source: quizlet.com
Rank the following atoms in order of the largest to smallest atomic radius: Of the following, which atom has the smallest atomic radius? Because of the charge present on it.
A) na b) al c) n d) f. Atomic radii vary in a predictable way across the periodic table. K+ has a larger atomic radius than na+.
 Source: chegg.com
Source: chegg.com
Al, p, cl, k k>al>p>cl below are data on the first four ionization energies for the fictitious element x This is due to trends in the periodic table, and the effective nuclear charge that holds the valence electrons close to the nucleus. Which of the following atoms has the smallest radius?
 Source: clutchprep.com
Source: clutchprep.com
Which of the following correctly lists the five atoms in order of the five atoms in order of increasing size (smallest to largest?) Which has largest radius co 3? Ionic radius of mn3+ will be largest.
 Source: clutchprep.com
Source: clutchprep.com
Which atom or ion has the smallest radius? A) rb b) si c) s d) o. Which has largest radius co 3?
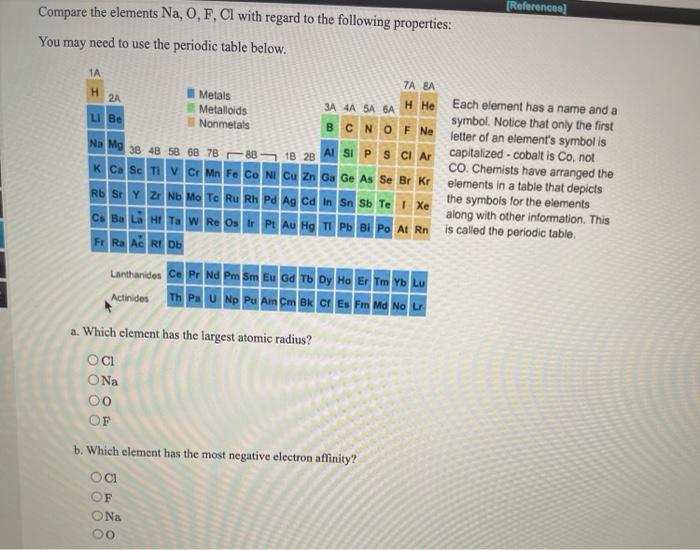
Of the following, which atom has the smallest atomic radius? So, all these elements belong to the 4th group and kr has the smallest atomic radii as it lies across the period. K+ has a larger atomic radius than na+.

Thus, helium is the smallest. One such trend involves the atomic radius for these elements. The atomic emission spectra of a sodium atom on earth and of a sodium atom in the sun would be;
 Source: clutchprep.com
Source: clutchprep.com
Al3+ has the smallest size. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top. A) na b) ba c) ca d) cs.
 Source: angelo.edu
Source: angelo.edu
The concentration of more protons in the nucleus creates a higher effective nuclear charge, in other words, there is a stronger force of attraction pulling the electrons closer to the nucleus resulting in a smaller atomic radius. Atomic radius generally increases as we move _____. Helium has the smallest atomic radius.
 Source: youtube.com
Source: youtube.com
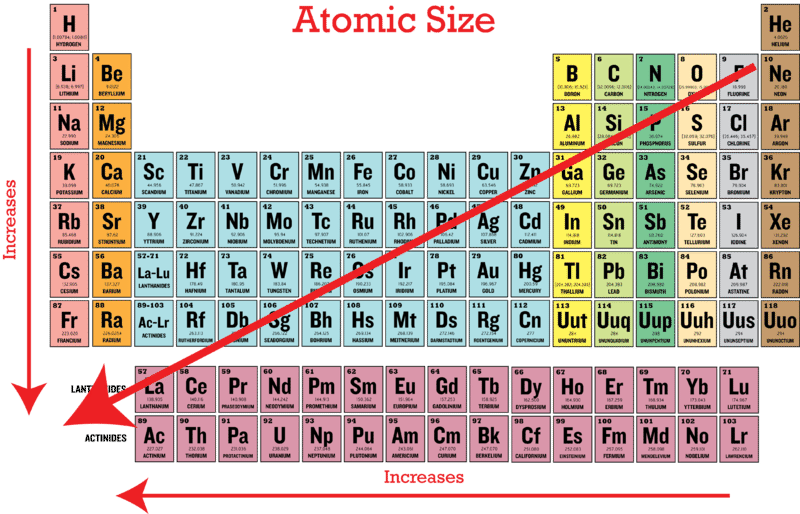
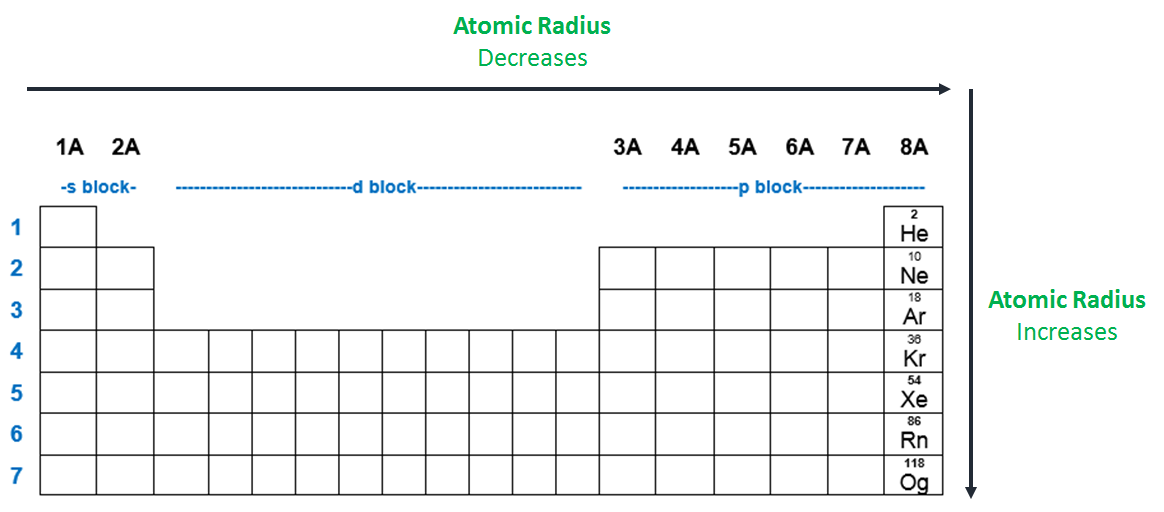
This atomic mass represents the; As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Which of the following atoms has the smallest radius?
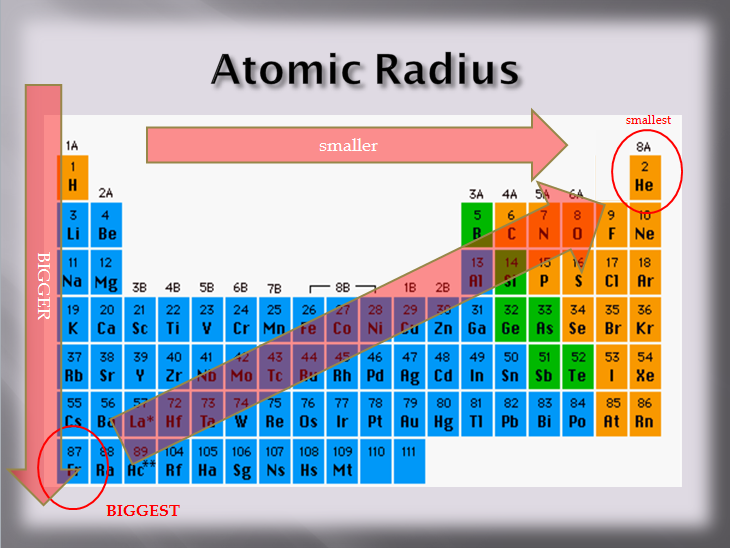 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
Heliumas can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Thus, helium is the smallest element, and francium is the largest. Atomic radius generally increases as we move _____.
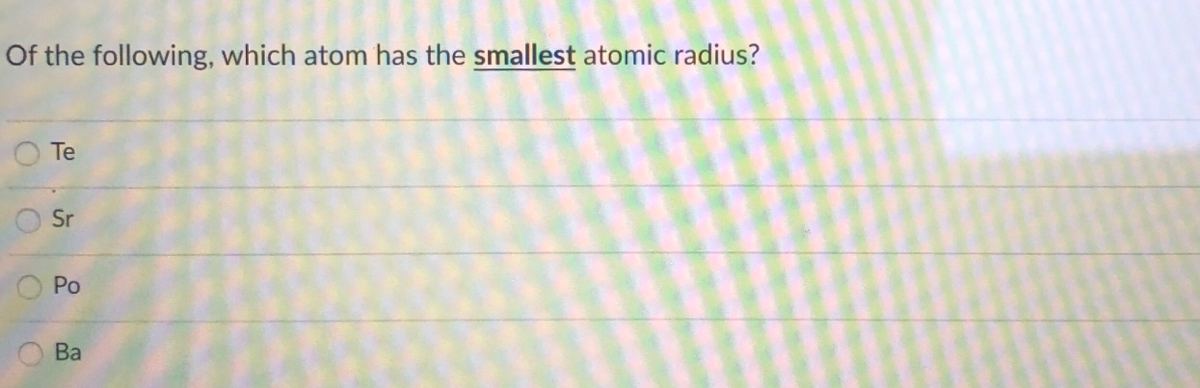 Source: bartleby.com
Source: bartleby.com
Which atom or ion has the smallest radius? This homework question can be solved by thinking about how the charges react to each other. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top.

Which atom or ion has the smallest radius? Which of the transition metals has the smallest atomic radius; Which of the following atoms has the smallest radius?
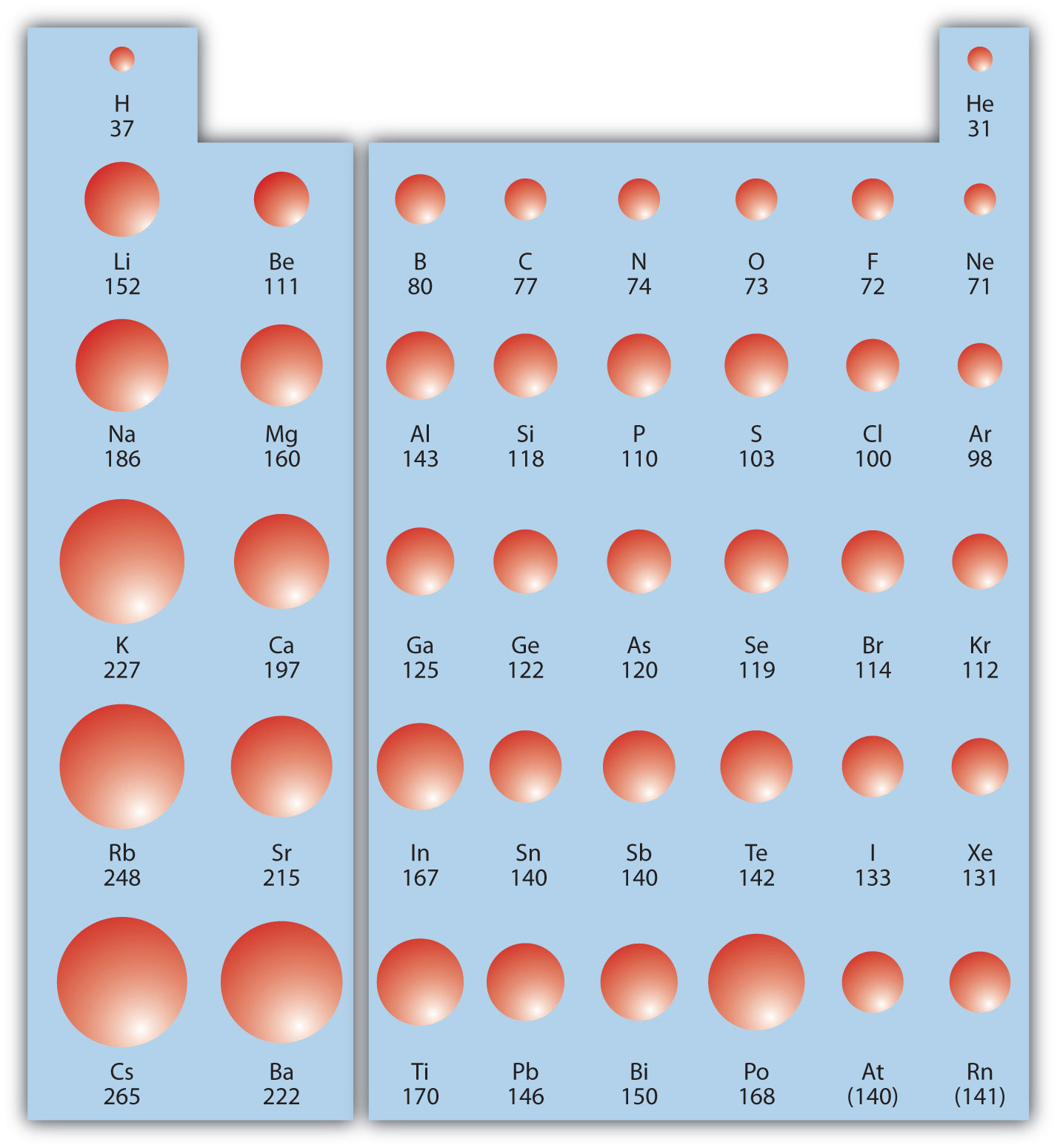 Source: socratic.org
Source: socratic.org
Heliumhelium has the smallest atomic radius. Franciumatomic radii vary in a predictable way across the periodic table. As the nuclear charge increases the attractive force between nucleus and electrons increases.
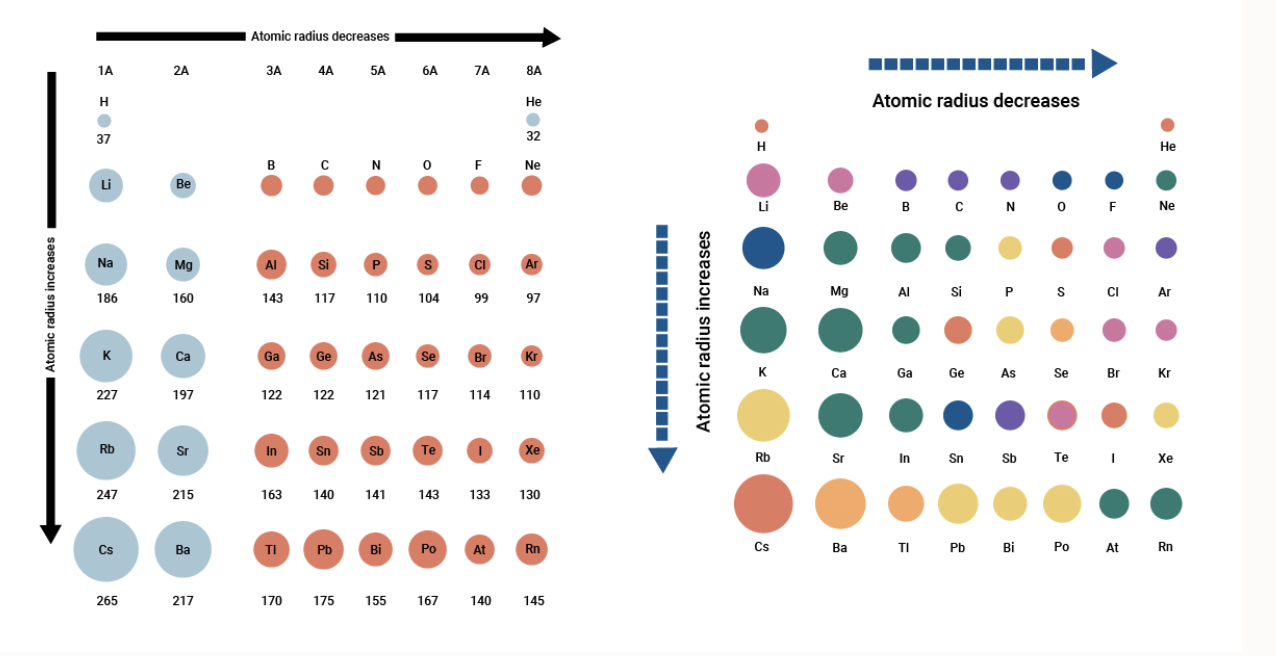 Source: breakingatom.com
Source: breakingatom.com
Of the following which gives the current order for the atomic radius for mg, na, p, si, and ar? The concentration of more protons in the nucleus creates a higher effective nuclear charge, in other words, there is a stronger force of attraction pulling the electrons closer to the nucleus resulting in a smaller atomic radius. As the nuclear charge increases the attractive force between nucleus and electrons increases.
 Source: blog.prepscholar.com
Source: blog.prepscholar.com
Atomic radii vary in a predictable way across the periodic table. As the nuclear charge increases the attractive force between nucleus and electrons increases. One such trend involves the atomic radius for these elements.
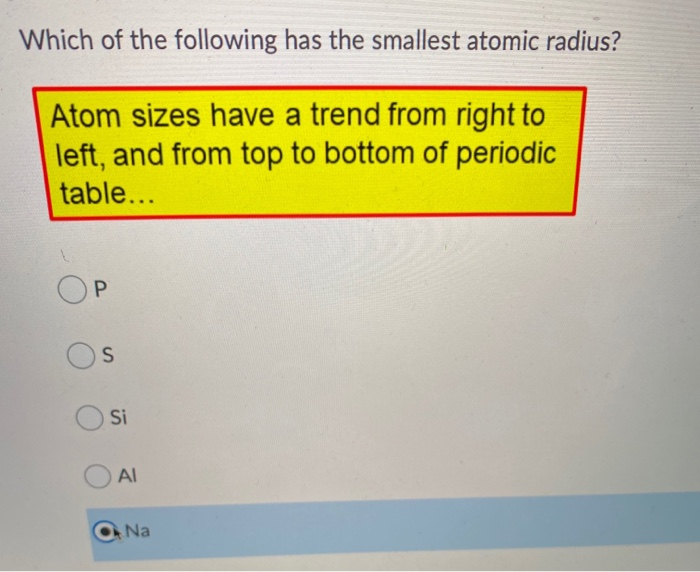 Source: chegg.com
Source: chegg.com
Helium has the smallest atomic radius. Al, p, cl, k k>al>p>cl below are data on the first four ionization energies for the fictitious element x Helium has the smallest atomic radius.
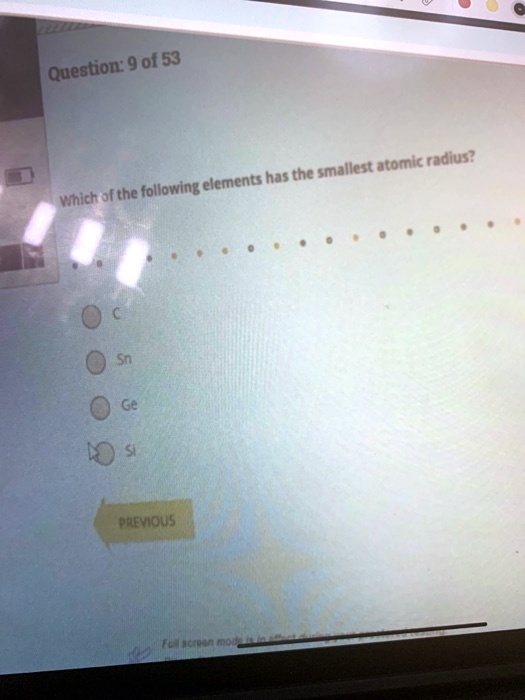 Source: numerade.com
Source: numerade.com
This atomic mass represents the; Atomic radii vary in a predictable way across the periodic table. Which atom or ion has the smallest radius?
 Source: chegg.com
Source: chegg.com
Thus, helium is the smallest element, and francium is the largest. A) rb b) si c) s d) o. As a result orbital electrons become more closer to nucleus.
 Source: chem.libretexts.org
Source: chem.libretexts.org
This is due to trends in the periodic table, and the effective nuclear charge that holds the valence electrons close to the nucleus. Since, the element which will in the right most of the theriodic table will be having smallest alomic radius. One such trend involves the atomic radius for these elements.
Also Read :