In h2o, there are two hydrogens present with oxidation states of plus one, and oxygen has an oxidation state of minus two. Oxidation refers to a chemical reaction that involves electron movement between the elements of any compound.
In Which Compound Is The Oxidation State Of Oxygen. In peroxides, such as hydrogen peroxide, h2o2 , each hydrogen has +1 charge, to give. In hydrogen peroxide, each hydrogen still has an oxidation number of +1 because each hydrogen “gives up” a single electron to oxygen. Learn more about how to calculate oxidation number along with the steps. Oxygen being less electronegative atom will have a positive oxidation state.
 How To Find Oxidation Numbers: 12 Steps (With Pictures) - Wikihow From wikihow.com
How To Find Oxidation Numbers: 12 Steps (With Pictures) - Wikihow From wikihow.com
Related Post How To Find Oxidation Numbers: 12 Steps (With Pictures) - Wikihow :
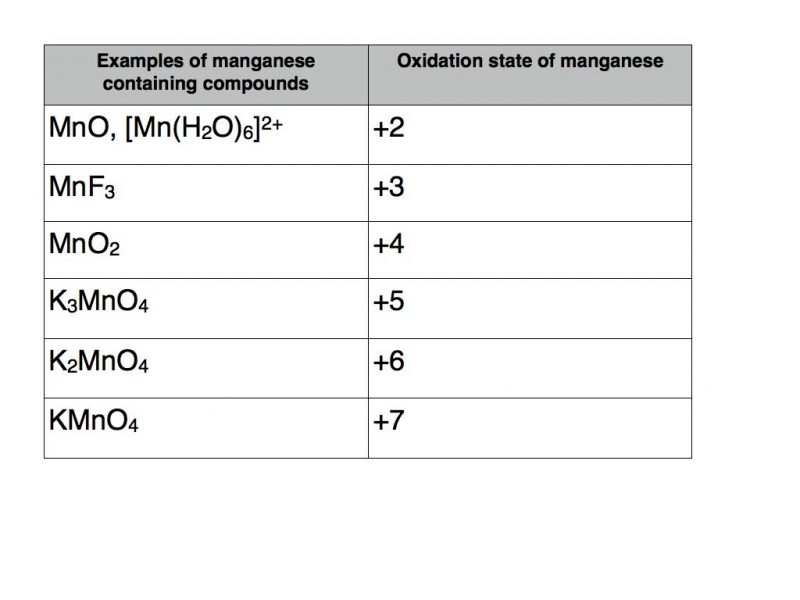
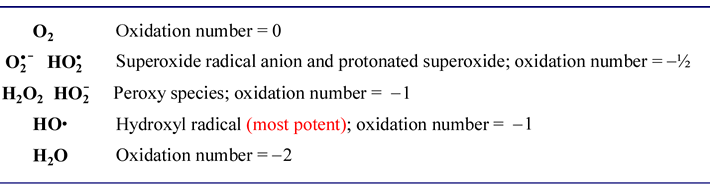
Substances obtained by the interaction of a chemical element with oxygen are called oxides. − 1 ⁄ 2 ( superoxides ), − 1 ⁄ 3 ( ozonides ), 0 (elemental, hypofluorous acid ), + 1 ⁄ 2 ( dioxygenyl ), +1 ( dioxygen difluoride ), and +2 ( oxygen difluoride ). Normally oxygen shows an oxidation of —2 in normal oxides. In peroxides, such as hydrogen peroxide, h2o2 , each hydrogen has +1 charge, to give.
In which compound does bromine have the highest oxidation state?
The problem here is that oxygen isn�t the most electronegative element. Compounds containing oxygen in other oxidation states are very uncommon: Oxidation is defined as the increase in the number of oxidation state. The oxidation state of oxygen is −2 in almost all known compounds of oxygen. Learn more about how to calculate oxidation number along with the steps. The primary difference between a peroxide and a superoxide lies in the oxidation state of the oxygen atom.
 Source: onlinechemistrytutor.net
Source: onlinechemistrytutor.net
A) hbro4 b) hbro3 c) brcl5 d) hbr. The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. Oxidation refers to a chemical reaction that involves electron movement between the elements of any compound.
 Source: onlinechemistrytutor.net
Source: onlinechemistrytutor.net
Due to its high electronegativity, oxygen usually has a negative two charge. In a compound or ion, the sum of the oxidation states equals the total charge of the compound or ion. Overall, the sum of the oxidation states will be zero for this neutral compound.
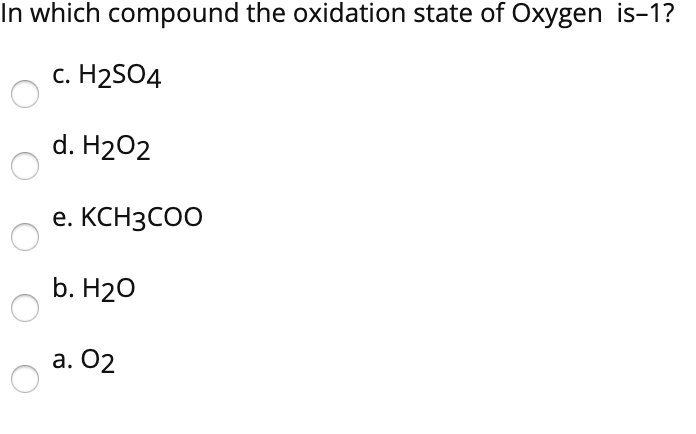 Source: chegg.com
Source: chegg.com
In hydrogen peroxide, each hydrogen still has an oxidation number of +1 because each hydrogen “gives up” a single electron to oxygen. Overall, the sum of the oxidation states will be zero for this neutral compound. Oxidation states refer to atoms or ions, not compounds.
 Source: youtube.com
Source: youtube.com
Learn more about how to calculate oxidation number along with the steps. In a compound or ion, the sum of the oxidation states equals the total charge of the compound or ion. It is also denoted by an increased oxidation state.
 Source: shutterstock.com
Source: shutterstock.com
Due to its high electronegativity, oxygen usually has a negative two charge. The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. It is also denoted by an increased oxidation state.
 Source: onlinechemistrytutor.net
Source: onlinechemistrytutor.net
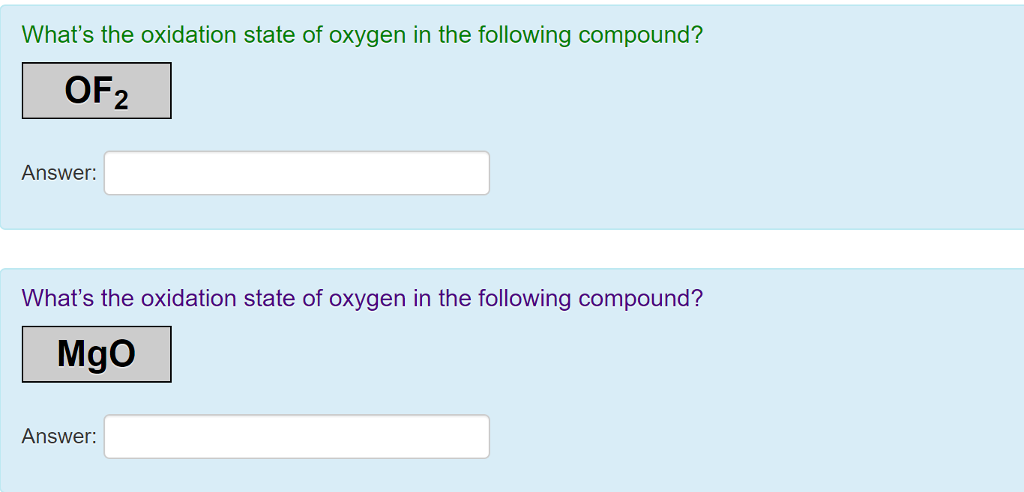
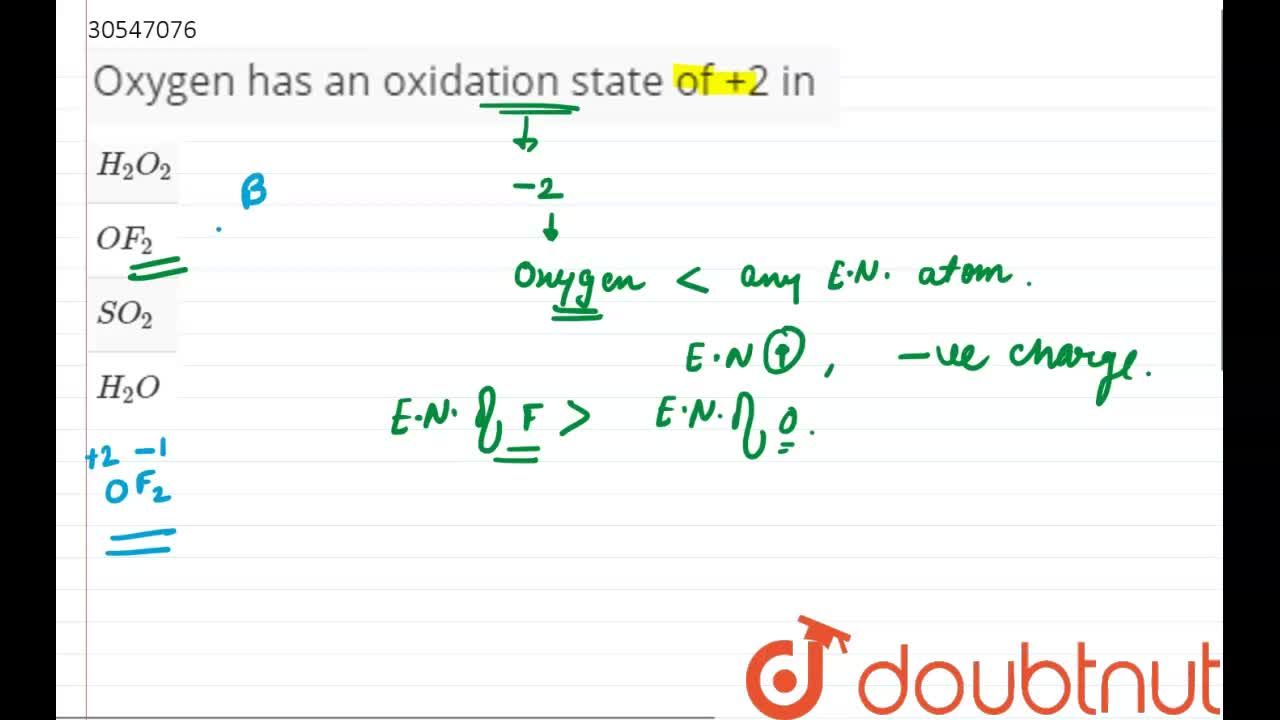
Oxygen in f 2 o. Oxygen has an oxidation number of +2 because the single oxygen atom has “gained” a total of two electrons, one from each hydrogen. Oxygen in f 2 o.
 Source: youtube.com
Source: youtube.com
Oxidation refers to a chemical reaction that involves electron movement between the elements of any compound. Oxygen being less electronegative atom will have a positive oxidation state. Due to its high electronegativity, oxygen usually has a negative two charge.
 Source: guweb2.gonzaga.edu
Source: guweb2.gonzaga.edu
Oxygen in f 2 o. Normally oxygen shows an oxidation of —2 in normal oxides. The problem here is that oxygen isn�t the most electronegative element.
 Source: brainly.in
Source: brainly.in
In peroxides, such as hydrogen peroxide, h2o2 , each hydrogen has +1 charge, to give. In which compound does bromine have the highest oxidation state? The oxidation state −1 is found in a few compounds such as peroxides.
![Solved] What Is The Oxidation State Of The Oxygen Atoms In Co2, O2 And H2O And What Does This Information Tell You About Photosynthesis And Respirat… | Course Hero](https://www.coursehero.com/qa/attachment/13691469/ “Solved] What Is The Oxidation State Of The Oxygen Atoms In Co2, O2 And H2O And What Does This Information Tell You About Photosynthesis And Respirat… | Course Hero”) Source: coursehero.com
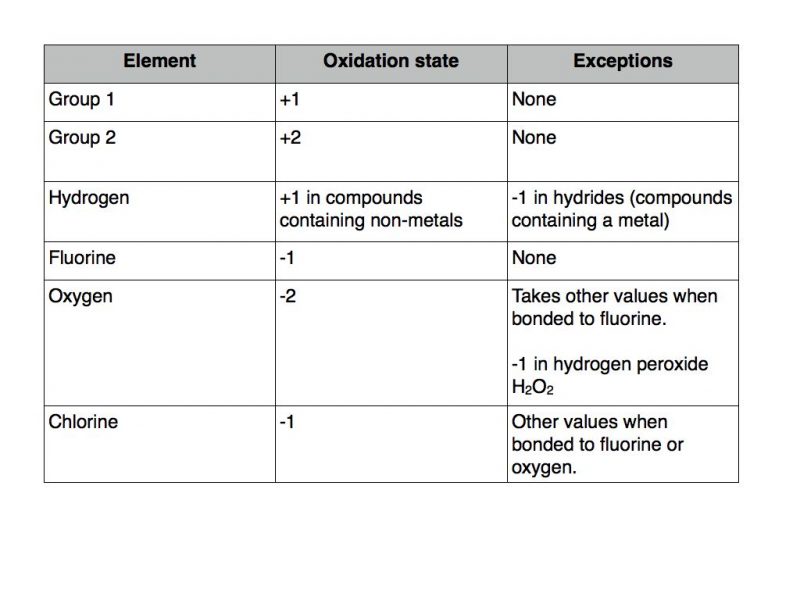
Oxidation refers to a chemical reaction that involves electron movement between the elements of any compound. All alkali metals (group 1 elements) have an oxidation state of +1 in their compounds. Normally oxygen shows an oxidation of —2 in normal oxides.
 Source: chegg.com
Source: chegg.com
In a compound or ion, the sum of the oxidation states equals the total charge of the compound or ion. Oxygen most often has an oxidation state of minus two, for example, in the compound water. Oxidation is defined as the increase in the number of oxidation state.

Hydrogen peroxide is an important compound of hydrogen and oxygen. However, we find that oxygen does sometimes take on other oxidation states in. Oxygen most often has an oxidation state of minus two, for example, in the compound water.
 Source: chemizzy.weebly.com
Source: chemizzy.weebly.com
A common example of oxidation is the reaction of iron (fe) with oxygen (o2). Oxidation states refer to atoms or ions, not compounds. In all superoxides ( k o x 2, c s o x 2, r b o x 2 ), oxygen has an oxidation state of − 1 2 ,this is because k, c s, r b, being elements of the first group and less electronegative than oxygen acquire a charge of + 1, to balance it, each oxygen atom acquires a charge of − 1 2.
 Source: wikihow.com
Source: wikihow.com
The oxidation state −1 is found in a few compounds such as peroxides. In which compound does bromine have the highest oxidation state? − 1 ⁄ 2 ( superoxides ), − 1 ⁄ 3 ( ozonides ), 0 (elemental, hypofluorous acid ), + 1 ⁄ 2 ( dioxygenyl ), +1 ( dioxygen difluoride ), and +2 ( oxygen difluoride ).
 Source: chegg.com
Source: chegg.com
In which compound does bromine have the highest oxidation state? Oxidation states refer to atoms or ions, not compounds. In which compound does bromine have the highest oxidation state?
 Source: nagwa.com
Source: nagwa.com
In h2o, there are two hydrogens present with oxidation states of plus one, and oxygen has an oxidation state of minus two. (a) o2 (b) h2o (c) h2so4 (d) h2o2 (e) kch3coo. This extends to chlorine and bromine only when not bonded to a lighter halogen, oxygen or nitrogen.
 Source: doubtnut.com
Source: doubtnut.com
All alkali metals (group 1 elements) have an oxidation state of +1 in their compounds. All alkali metals (group 1 elements) have an oxidation state of +1 in their compounds. Click to see full answer regarding this, why is the oxidation number of oxygen?

Oxygen being less electronegative atom will have a positive oxidation state. Oxygen in f 2 o. But in case of peroxides ,oxygen shows an oxidation state of —1 as we can see in h2o2 or na2o2.
 Source: sciencenotes.org
Source: sciencenotes.org
(a) o2 (b) h2o (c) h2so4 (d) h2o2 (e) kch3coo. The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. In a compound or ion, the sum of the oxidation states equals the total charge of the compound or ion.
 Source: youtube.com
Source: youtube.com
Oxgyen also shows positive oxidation state when it combines with flourine. It is also denoted by an increased oxidation state. Hydrogen peroxide is an important compound of hydrogen and oxygen.
Also Read :