Infact, it has multiple oxidation state to a maximum of +7. People also ask, which compound has the atom with the highest oxidation number?
In Which Compound Does Chlorine Have The Highest Oxidation Number. But chlorine can have other oxidation numbers, for example: Since the algebraic sum of the oxidation number of all atoms in a compound must be zero. A) naclo3 b) naclo c) naclo 4 d) naclo2 2) in which substance does chlorine have an oxidation number of +1? In per chloric acid hclo4, the oxidation number of chlorine is +7.

Related Post Solved In Which One Of The Following Compounds Or Ions Does | Chegg.com :
Oxidation numbers also play an important role in the systematic nomenclature of chemical compounds. The oxidation number of a monatomic ion equals the charge of the ion. Iron(iii) chloride contains iron with an oxidation number. − 2 + x = − 1 o r x = + 1 d.
Hclo 4, cl 2 o 7 are examples for +7 oxidation number of chlorine.
In a compound, hydrogen has an oxidation number of +1. 1) in which compound does chlorine have the highest oxidation number? Infact, it has multiple oxidation state to a maximum of +7. In which compound does manganese have the highest oxidation state? Iron(iii) chloride contains iron with an oxidation number. A) kcio b) kcio2 c) kcio3 d) kcio4 i�m really stuck on these problem so please don�t just tell me the answer i know its 4 but how do you get it, how do you find the oxidation number in situations like this??

- in which substance does chlorine have an oxidation number of +1? The oxidation number of a free element is always 0. Infact, it has multiple oxidation state to a maximum of +7.

Yes you are right sulphur has the highest oxidation number. Of the reactions below, only is not spontaneous. As you can see in the periodic table of elements, the halogens that are good oxidizing agents are fluorine, chlorine, bromine and iodine, with fluorine being the strongest oxidizing agent among the four, followed by chlorine, bromine and iodine.
 Source: numerade.com
Source: numerade.com
The oxidation number of c l in the given compounds is as shown below. Of the reactions below, only is not spontaneous. But chlorine can have other oxidation numbers, for example:
 Source: onlinechemistrytutor.net
Source: onlinechemistrytutor.net
The oxidation number of a free element is always 0. The oxidation number of c l in the given compounds is as shown below. The oxidation state, often called the oxidation number, is an indicator of the degree of oxidation (loss of electrons) of an atom in a chemical compound.
 Source: slideplayer.com
Source: slideplayer.com
C c l 4 is covalent and thus oxidation of all c l is same. People also ask, which compound has the atom with the highest oxidation number? Conceptually, the oxidation state, which may be positive, negative or zero, is.
 Source: yumpu.com
Source: yumpu.com
The oxidation number of c l in the given compounds is as shown below. A) cl2 b) hcl c) hclo 2 d) hclo 3) what is the oxidation number assigned to manganese in kmno4? By definition, the oxidation number of an atom is the charge that atom would have if the compound was composed of ions.
 Source: compoundchem.com
Source: compoundchem.com
- what is the oxidation number assigned to manganese in. In which species does sulfur have the highest oxidation number a) sg (elemental form of sulfur) b) h2s c) so2 d) h2s03 e) k2s04 9. What is the oxidation state of manganese in kmno4?
 Source: studylib.net
Source: studylib.net
How do you find the oxidation number of a transition metal? In which species does sulfur have the highest oxidation number a) sg (elemental form of sulfur) b) h2s c) so2 d) h2s03 e) k2s04 9. Additionally, what is the oxidation number of chlorine in naclo?

By definition, the oxidation number of an atom is the charge that atom would have if the compound was composed of ions. 3) what is the oxidation number assigned to manganese in. _____ 1) in which compound does chlorine have the highest oxidation number?.

Hclo 4, cl 2 o 7 are examples for +7 oxidation number of chlorine. Additionally, what is the oxidation number of chlorine in naclo? Oxidation numbers also play an important role in the systematic nomenclature of chemical compounds.
 Source: toppr.com
Source: toppr.com
A) kcio b) kcio2 c) kcio3 d) kcio4 i�m really stuck on these problem so please don�t just tell me the answer i know its 4 but how do you get it, how do you find the oxidation number in situations like this?? So the oxidation number of the compound hcl is zero. (1) naclo (3) naclo 3 (2) naclo 2 (4) naclo 4.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Thus, the highest oxidation number of chlorine is in kclo4. (1) +7 (3) +3 (2) +2 (4) +4. What is the oxidation state of manganese in mno4?
 Source: doubtnut.com
Source: doubtnut.com
Oxidation numbers also play an important role in the systematic nomenclature of chemical compounds. Conceptually, the oxidation state, which may be positive, negative or zero, is. A) magnesiun b) zinc c) chromium d) iron e) nickel 10.
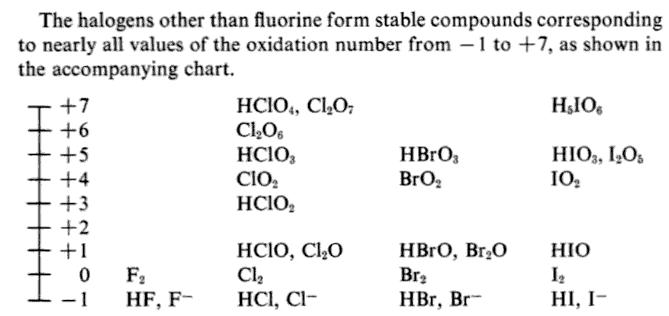 Source: socratic.org
Source: socratic.org
To answer your second question, chlorine can fill its valence with a +2 oxidation state. Follow 6 answers report abuse answers relevance sara. What is the oxidation number of carbon in ch2cl2?

In which species does sulfur have the highest oxidation number a) sg (elemental form of sulfur) b) h2s c) so2 d) h2s03 e) k2s04 9. Conceptually, the oxidation state, which may be positive, negative or zero, is. Thus, the highest oxidation number of chlorine is in kclo4.
 Source: amp.doubtnut.app
Source: amp.doubtnut.app
How do you find the oxidation number of a transition metal? − 2 + x = − 1 o r x = + 1 d. Yes you are right sulphur has the highest oxidation number.
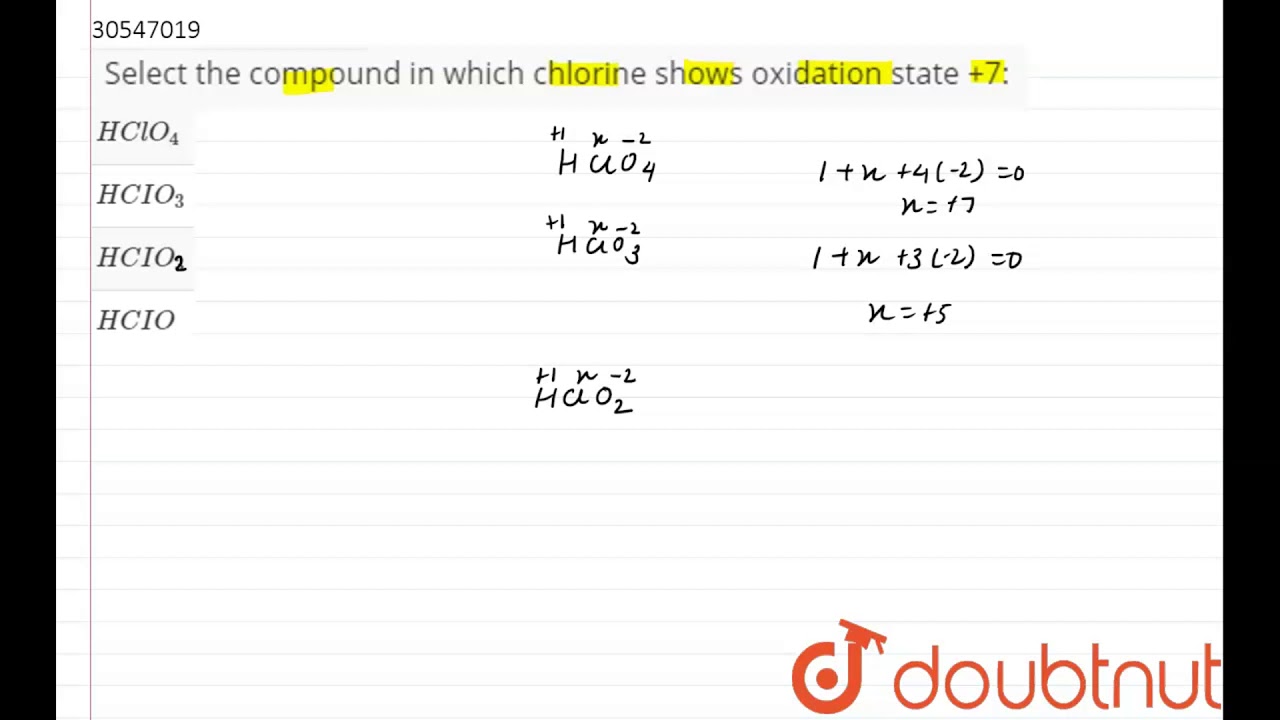 Source: youtube.com
Source: youtube.com
The oxidation number of a monatomic ion equals the charge of the ion. In a compound, hydrogen has an oxidation number of +1. To see more answers head over to college study.
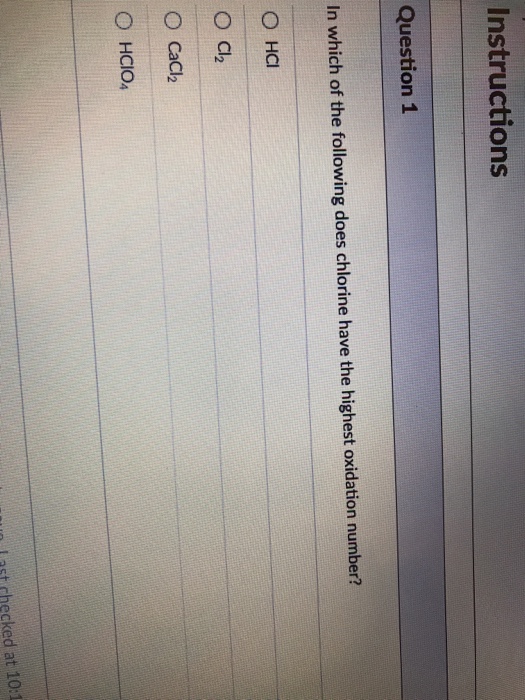
Because transition metals have more than one stable oxidation state, we use a number in roman numerals to indicate the oxidation number e.g. By definition, the oxidation number of an atom is the charge that atom would have if the compound was composed of ions. In which compound does chlorine have the highest oxidation number?
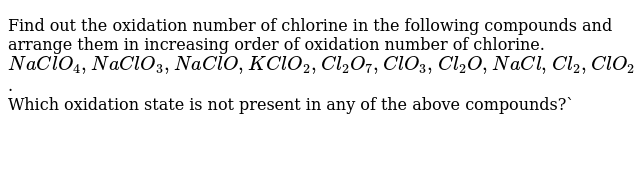 Source: amp.doubtnut.app
Source: amp.doubtnut.app
In per chloric acid hclo4, the oxidation number of chlorine is +7. A) kcio b) kcio2 c) kcio3 d) kcio4 i�m really stuck on these problem so please don�t just tell me the answer i know its 4 but how do you get it, how do you find the oxidation number in situations like this?? In a compound, hydrogen has an oxidation number of +1.
 Source: youtube.com
Source: youtube.com
Out of 4, manganese in limno4 has the highest oxidation number. So the oxidation number of the compound hcl is zero. Sum of all the oxidation numbers in a polyatomic ion is equal to the charge on the ion.
Also Read :





