Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds. Covalent compounds tend to be more flammable that ionic compounds.
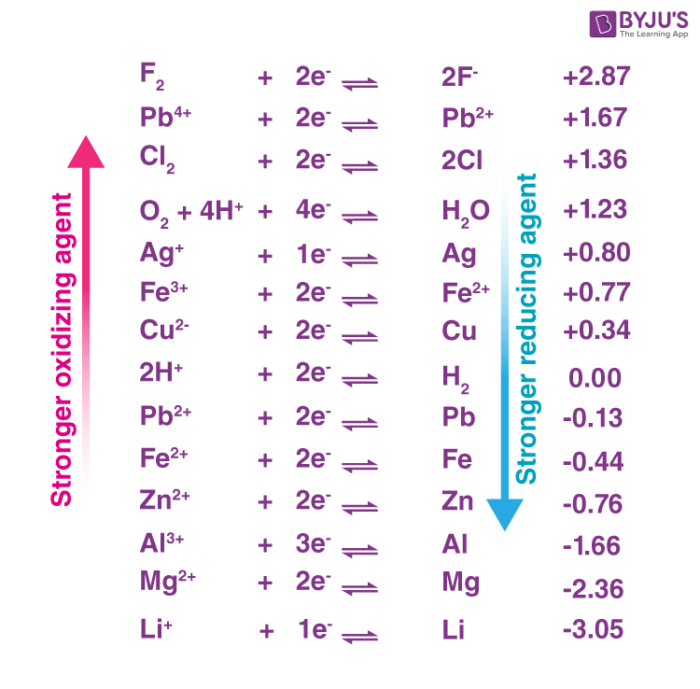
Covalent Compounds Display Which Of These Properties. Superior malleability is not a property of covalent compounds. They are inefficient electrical and thermal conductors. (d) boiling and melting point of carbon compounds are relatively lower than those of ionic compounds ans : Covalent compounds are generally poor conductors of electricity.
 Ch150: Chapter 4 – Covalent Bonds And Molecular Compounds – Chemistry From wou.edu
Ch150: Chapter 4 – Covalent Bonds And Molecular Compounds – Chemistry From wou.edu
Related Post Ch150: Chapter 4 – Covalent Bonds And Molecular Compounds – Chemistry :
Due to covalent bonds, their boiling and melting points are relatively lower than those. (a) carbon compounds are good conductor of heat and electricity carbon compounds are covalently bonded and are poor conductor of heat and electricity. In a covalent compound, the covalent molecules are held together by weak forces of attraction. It is soluble in water, ammonia, acid but insoluble in alcohol.
They are hard solids consisting of ions.
Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds. Most covalent compounds have relatively low melting points and boiling points. (ii) these compounds are generally solids. The intermolecular attractions are weak which require a small amount of energy. Compounds formed by covalent bonding don’t conduct electricity due to the lack of free electrons. Most covalent compounds have relatively low melting points and boiling points.
 Source: chemistrytalk.org
Source: chemistrytalk.org
As with many properties, there are exceptions, primarily when molecular compounds assume crystalline forms. The compounds containing covalent bonds are called covalent compounds. The intermolecular attractions are weak which require a small amount of energy.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Solids are brittle and variously coloured. At the same time, the nitrogen and hydrogen atoms have covalent bonds. It has a boiling point of 3,600 °c and a melting point of 2,852 °c.
 Source:
Source:
Covalent compounds tend to be more flammable than ionic compounds. While the ions in an ionic compound are strongly attracted to each other, covalent bonds create molecules that can separate from each other when a lower amount of energy is added to them. These can be easily overcome by heat.
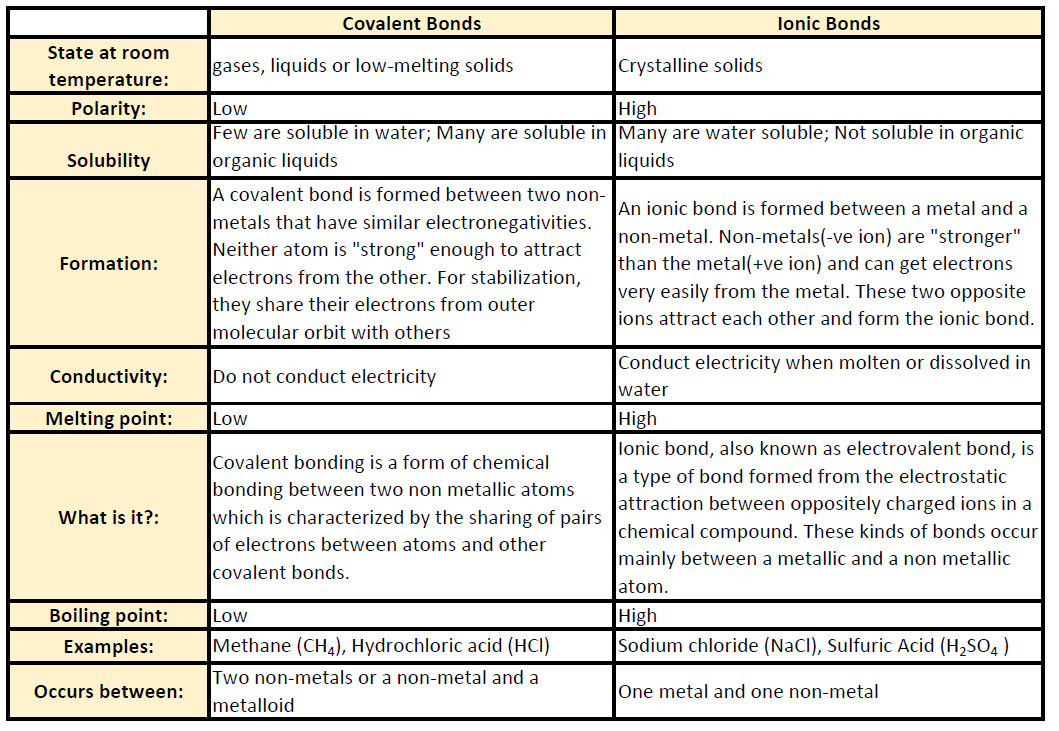 Source: wou.edu
Source: wou.edu
Certain properties possessed by these compounds differ because of differences in the types of bonds present in these compounds. Cl 6 i 2 cas registry number: Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds.
 Source: toppr.com
Source: toppr.com
Covalent compounds are generally poor conductors of electricity. Following are the typical characteristics of covalent compounds: Covalent and ionic compounds are held by covalent and ionic bonds respectively.

Due to weak intermolecular forces, they are easy to distort or break. Covalent and ionic compounds are held by covalent and ionic bonds respectively. Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds.
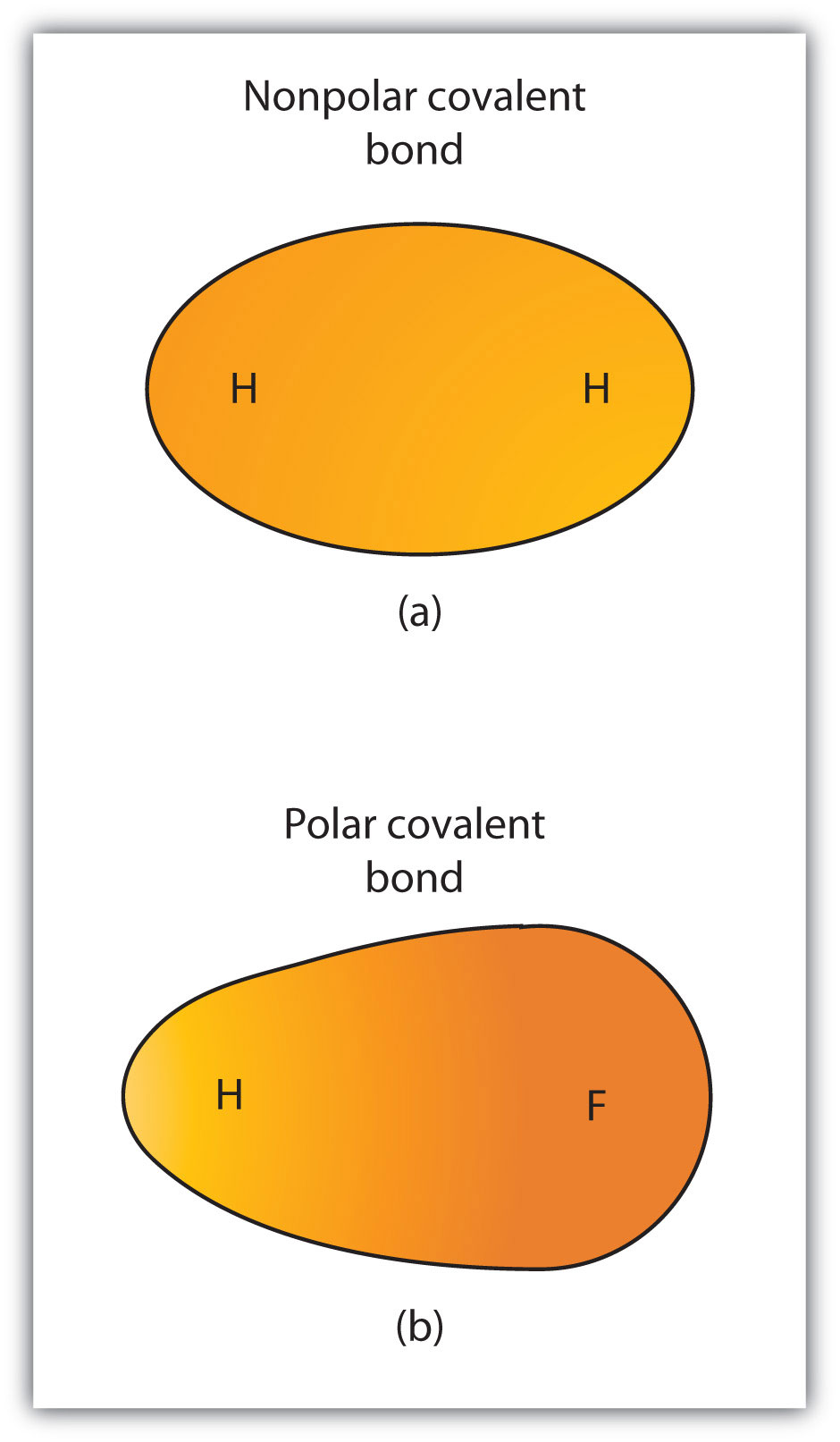 Source: wou.edu
Source: wou.edu
They are good conductors of electricity in fused state. Thus, covalent compounds have low melting points and low boiling points. Following are the properties of covalent compounds :
 Source: wou.edu
Source: wou.edu
Covalent compounds consist of molecules held by weak forces. Compounds formed by covalent bonding don’t conduct electricity due to the lack of free electrons. While the ions in an ionic compound are strongly attracted to each other, covalent bonds create molecules that can separate from each other when a lower amount of energy is added to them.
 Source: wou.edu
Source: wou.edu
Covalent compounds are soft and squishy. Hence, the covalent compound has low melting and boiling points with high volatility. Covalent and ionic compounds are held by covalent and ionic bonds respectively.
 Source: slideplayer.com
Source: slideplayer.com
Most covalent compounds have relatively low melting points and boiling points. Covalent and ionic compounds are held by covalent and ionic bonds respectively. It is soluble in water, ammonia, acid but insoluble in alcohol.

(a) carbon compounds are good conductor of heat and electricity carbon compounds are covalently bonded and are poor conductor of heat and electricity. Covalent compounds tend to be more flammable than ionic compounds. [icl 3] 2 hill system formula:
 Source: britannica.com
Source: britannica.com
These are gases or liquids or soft solids. Thus, covalent compounds have low melting points and low boiling points. Solids are brittle and variously coloured.
 Source: wou.edu
Source: wou.edu
Covalent compounds tend to be soft and relatively flexible. Following are the properties of covalent compounds : Covalent compounds are generally poor conductors of electricity.
 Source: sciencedirect.com
Source: sciencedirect.com
At the same time, the nitrogen and hydrogen atoms have covalent bonds. Covalent compounds are nonpolar, and water is a polar solvent. Low melting points and boiling points.
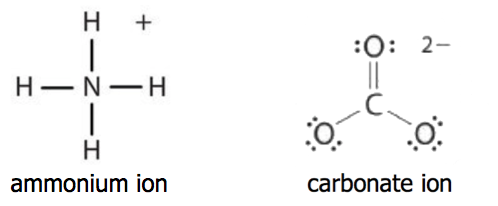 Source: chem.libretexts.org
Source: chem.libretexts.org
It has a boiling point of 3,600 °c and a melting point of 2,852 °c. They are good conductors of electricity in fused state. Covalent compounds tend to be more flammable than ionic compounds.
 Source: sciencedirect.com
Source: sciencedirect.com
Most compounds having covalent bonds exhibit relatively low melting points and boiling points. Following are the typical characteristics of covalent compounds: What is the octet rule?
 Source: wou.edu
Source: wou.edu
Covalent compounds usually have lower enthalpies of fusion and vaporization than ionic compounds. In covalent compounds (unlike ionic compounds), we can talk about independent molecules, as covalent compounds are composed of independent molecules linked together by different bonds (van derval, hydrogen) of varying strength. 101°c (16 atmospheres, 65°c decomposes) boiling point:
 Source: thoughtco.com
Source: thoughtco.com
Covalent compounds tend to be more flammable than ionic compounds. Due to covalent bonds, their boiling and melting points are relatively lower than those. A small amount of heat energy is required to overcome the weak intermolecular forces of attraction during melting or boiling.

Covalent compounds tend to be soft and relatively flexible. As with many properties, there are exceptions, primarily when molecular compounds assume crystalline forms. [icl 3] 2 hill system formula:
 Source:
Source:
(i) low melting and boiling points: What is the octet rule? Most covalent compounds have relatively low melting points and boiling points.
Also Read :