Molecules of each species that form (the products) are the same and are given by the chemical reaction equation. The radius of he clock face is 10 inches.
Balancing Chemical Reactions Is Consistent With Which Scientific Law. The face of a clock is divided into 12 equal parts. Suppose we have the balanced. Law of conversation of mass when the equation, ___o2 + ___c 6h 14 →→ ___co2 + ___h2o is. The na and o atoms are balanced—one na and one o on each side—but there are two h atoms on the left and three h atoms on the right.
 4.1 Writing And Balancing Chemical Equations – Chemistry From opentextbc.ca
4.1 Writing And Balancing Chemical Equations – Chemistry From opentextbc.ca
Related Post 4.1 Writing And Balancing Chemical Equations – Chemistry :
10 grams of reactant = 10 grams of products. Consistent with the law of. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter.
According to law of conservation of mass:
Balancing chemical equations is necessary due to the law of conservation of matter/mass, which states that in a chemical reaction matter is neither created nor destroyed. Balancing chemical reactions is consistent with which scientific law? They must obey the law of conservation of mass that states that matter cannot be created or destroyed, it is conserved. Balancing chemical reactions is not chemistry; Chemical reaction in which one substance breaks down into two or more substances. Mass of reactants = mass of products.
 Source: youtube.com
Source: youtube.com
Arrange the following salts according to their solubility in water. Therefore, a balanced chemical equation will show the same number of each type of atom on each side of the equation. Observe how much salt dissolves before the solution becomes saturated.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Mass of reactants = mass of products. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents.
 Source: numerade.com
Source: numerade.com
The law of conservation of mass is observed in a balanced chemical equation, which is a chemical equation that shows all mass is conserved throughout the reaction. Balancing of a chemical equation is based on the principle of atom conservation (poac) which states that the total number of atoms of each element in reactants must equal the number of atoms of that element in products. This is a requirement the equation must satisfy.
 Source: chegg.com
Source: chegg.com
The balanced equation will appear above. Balancing chemical reactions is consistent with which scientific law? The law of conservation of mass is observed in a balanced chemical equation, which is a chemical equation that shows all mass is conserved throughout the reaction.
 Source: australiancurriculum.edu.au
Source: australiancurriculum.edu.au
It is just linear algebra. The law of conservation of matter states that no atoms are created or destroyed in a chemical reaction. Hydrogen gas reacts with chlorine gas to form hydrogen chloride.
 Source: slideplayer.com
Source: slideplayer.com
The law of conservation of mass states that, for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as system�s mass cannot change, so quantity cannot be added nor removed. Balancing chemical reactions is not chemistry; Hence, it is proved that the law of conservation of mass is followed by the above reaction.
 Source: clutchprep.com
Source: clutchprep.com
The law of conservation of mass states that, for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as system�s mass cannot change, so quantity cannot be added nor removed. Balancing chemical reactions is consistent with which scientific law? A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products.
 Source: slideplayer.com
Source: slideplayer.com
Balance chemical equations by comparing the numbers of each type of atom in the reactants and products. 10 grams of reactant = 10 grams of products. Balancing chemical reactions is consistent with which scientific law?
 Source: chegg.com
Source: chegg.com
Why is balancing equations important? Atoms are never lost or gained in chemical reactions, they are rearranged. Thus, we need to increase the number of h atoms on the left.
 Source: chem.libretexts.org
Source: chem.libretexts.org
According to law of conservation of mass: Hydrogen gas reacts with chlorine gas to form hydrogen chloride. A chemical reaction, when it is feasible, is a natural process, the consequent
 Source: docbrown.info
Source: docbrown.info
The radius of he clock face is 10 inches. ∴ 10 gram of caco 3 = 3.8 grams of co 2 + 6.2 grams of cao. Making sure the default volume of water is 1.00×10−16 l when using the slightly soluble salts tab, add each of the possible salts to the water.
 Source: study.com
Source: study.com
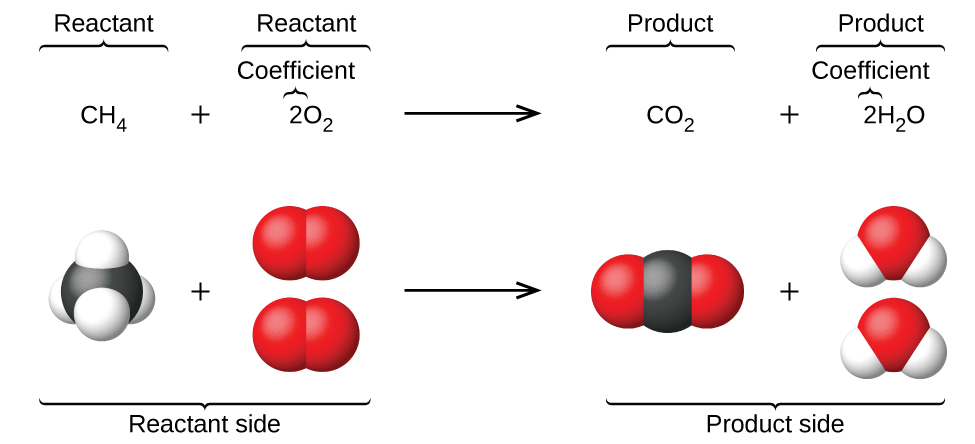
Process that changes one set of chemicals into another set of chemicals. The law of conservation of mass states that, for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as system�s mass cannot change, so quantity cannot be added nor removed. Balancing equations the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides.
 Source: studylib.net
Source: studylib.net
A chemical equation should be balanced to achieve the law of conservation of matter. The law of conservation of mass is observed in a balanced chemical equation, which is a chemical equation that shows all mass is conserved throughout the reaction. Hydrogen gas reacts with chlorine gas to form hydrogen chloride.
 Source: slideplayer.com
Source: slideplayer.com
Observe how much salt dissolves before the solution becomes saturated. Reaction stoichiometry could be computed for a balanced equation. The results of the equation balancing comply with the law on the conservation of matter and confirm that the existing methods for balance of chemical.

A chemical equation should be balanced to achieve the law of conservation of matter. Atoms are never lost or gained in chemical reactions, they are rearranged. Balancing chemical equations is necessary due to the law of conservation of matter/mass, which states that in a chemical reaction matter is neither created nor destroyed.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Suppose we have the balanced. According to law of conservation of mass: Solved balancing chemical reactions is consistent with which | chegg.com.
 Source: researchgate.net
Source: researchgate.net
This is a requirement the equation must satisfy. Why must chemical equations be balanced? The radius of he clock face is 10 inches.
 Source: study.com
Source: study.com
A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. Why must chemical equations be balanced? Process that changes one set of chemicals into another set of chemicals.
 Source: docbrown.info
Source: docbrown.info
Balancing equations the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. The law of conservation of mass is observed in a balanced chemical equation, which is a chemical equation that shows all mass is conserved throughout the reaction. Molecules of each species that form (the products) are the same and are given by the chemical reaction equation.
 Source: opentextbc.ca
Source: opentextbc.ca
They must obey the law of conservation of mass that states that matter cannot be created or destroyed, it is conserved. 20.00 g of aluminum (al) reacts with 78.78 grams of molecular chlorine (cl2) all of each reactant is completely consumed Ionic charges are not yet supported and will be ignored.
Also Read :





