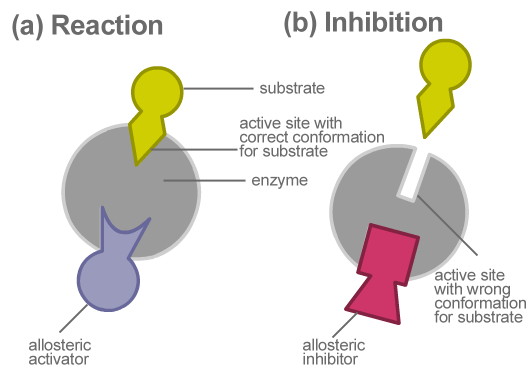
O after dissociation of allosteric inhibitor from enzyme, substrate can bind to enzyme at active site o binding of allosteric inhibitor to the active site on enzyme o binding of substrate to active site. Allosteric enzymes have active and inactive shapes differing in 3d structure.
An Allosteric Inhibitor Does Which Of The Following. Some examples of irreversible inhibitors include nerve gas,. The following statements are true for feedback allosteric inhibition in multienzyme system: The existence of allosteric sites on receptor molecules has expanded potential drug mechanisms. The enzyme substrate complex is maximal.
 Enzymes | Openstax Biology 2E From courses.lumenlearning.com
Enzymes | Openstax Biology 2E From courses.lumenlearning.com
Related Post Enzymes | Openstax Biology 2E :
Binds to an enzyme away from the active site and changes the conformation of the active site, increasing its affinity for substrate binding. The enzyme substrate complex is maximal. Binds to an enzyme away from the active site and changes the conformation of the active site, increasing its affinity for substrate binding. This causes a conformational change in the active site for the second molecule, preventing binding.
An allosteric inhibitor does which of the following?
Binds to an enzym… 00:49. This can be classified into the following types as. Allosteric enzymes enzymes with multiple subunits have quaternary structure. The allosteric activator binds to an enzyme at a site other than the active site. Binds to an enzym… 00:49. The increase in an enzymes activity that occurs when an allosteric activator binds to its specific regulatory site on the enzyme.

The inhibition of deoxythymidilate formation and subsequent blockage of cell division is due to a. The inhibition of deoxythymidilate formation and subsequent blockage of cell division is due to a. Amp diminishes and citrate enhances the inhibitory effect of atp.
 Source: chegg.com
Source: chegg.com
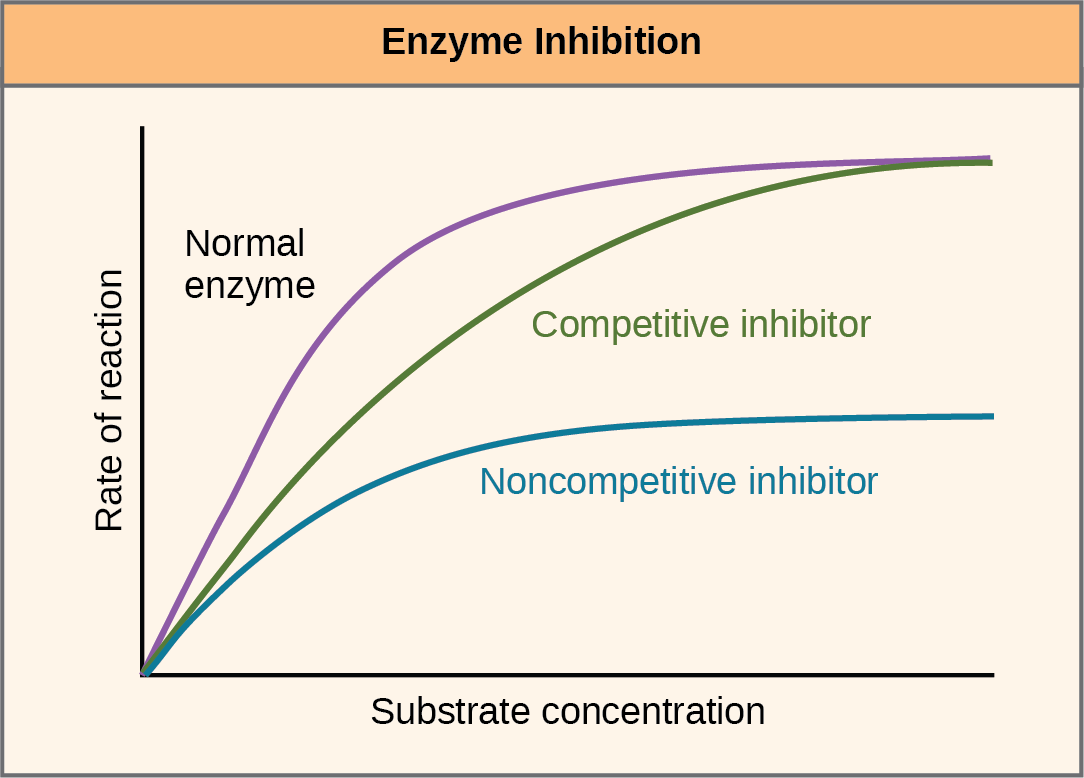
Which of the following graphs shows the results of reaction rate vs substrate concentration for an allosteric enzyme in the absence and presence of an allosteric inhibitor? When an allosteric inhibitor binds to an enzyme, all active sites on the protein subunits are changed slightly so that they work less well. Allosteric modulation or feedback inhibition is an enzyme regulatory mechanism where a product of a simple or chain reaction can function as a temporary allosteric inhibitor, i.e., an inhibitor that combines with a regulatory or allosteric site (other than the active site) if its concentration.
 Source: differencebetween.com
Source: differencebetween.com
This means that the affinity between enzyme and substrate is not altered in noncompetitive inhibition. Amp diminishes and citrate enhances the inhibitory effect of atp. An allosteric inhibitor does which of the following?
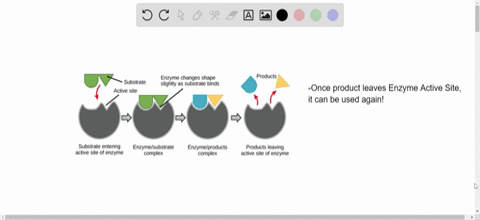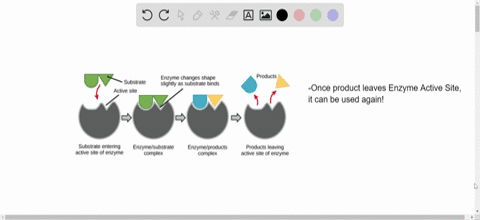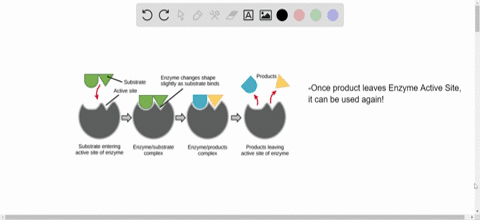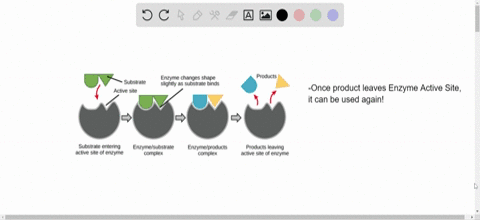 Source: numerade.com
Source: numerade.com
This causes a conformational change in the active site for the second molecule, preventing binding. The allosteric activator binds to an enzyme at a site other than the active site. The substrate concentration exceeds that of a noncompetitive inhibitor.
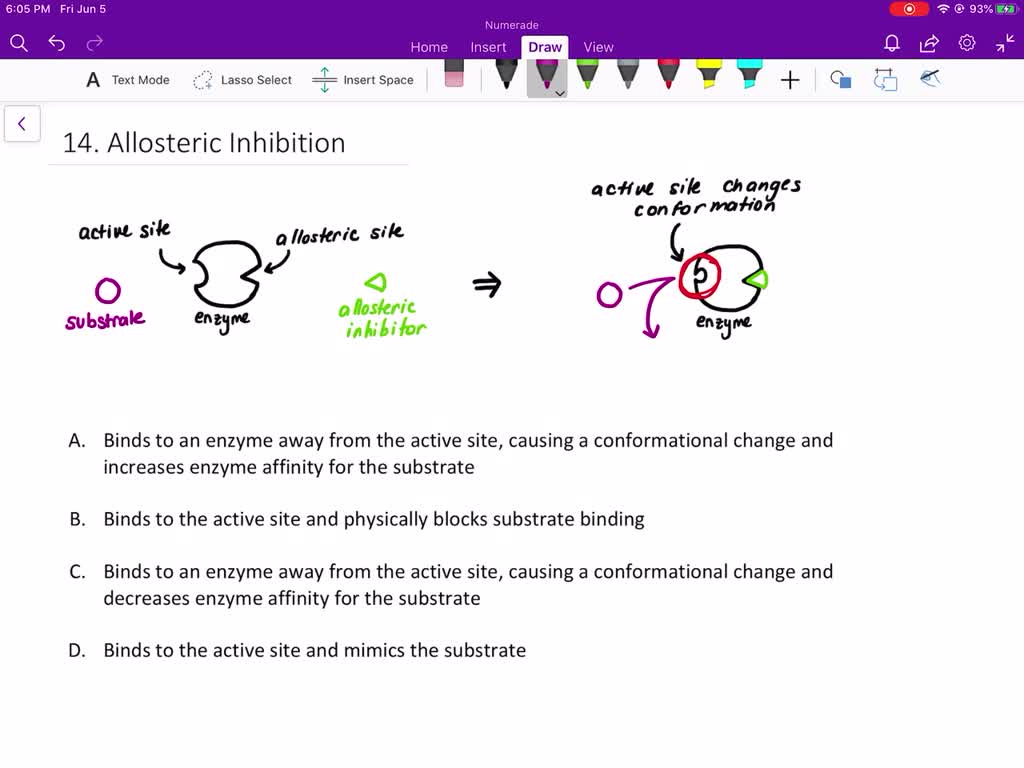 Source: numerade.com
Source: numerade.com
Allosteric enzymes are enzymes that have an additional binding site for effector molecules other than the active site. The substrate concentration is equal to that of a competitive inhibitor b. Allosteric enzymes typically have multiple active sites located on different protein subunits.

Allosteric inhibition is the type of enzymatic regulation where the inhibitor binds to a site other than the active site. The active site changes shape when an inhibitor binds to an allosteric site. The increase in an enzymes activity that occurs when an allosteric activator binds to its specific regulatory site on the enzyme.
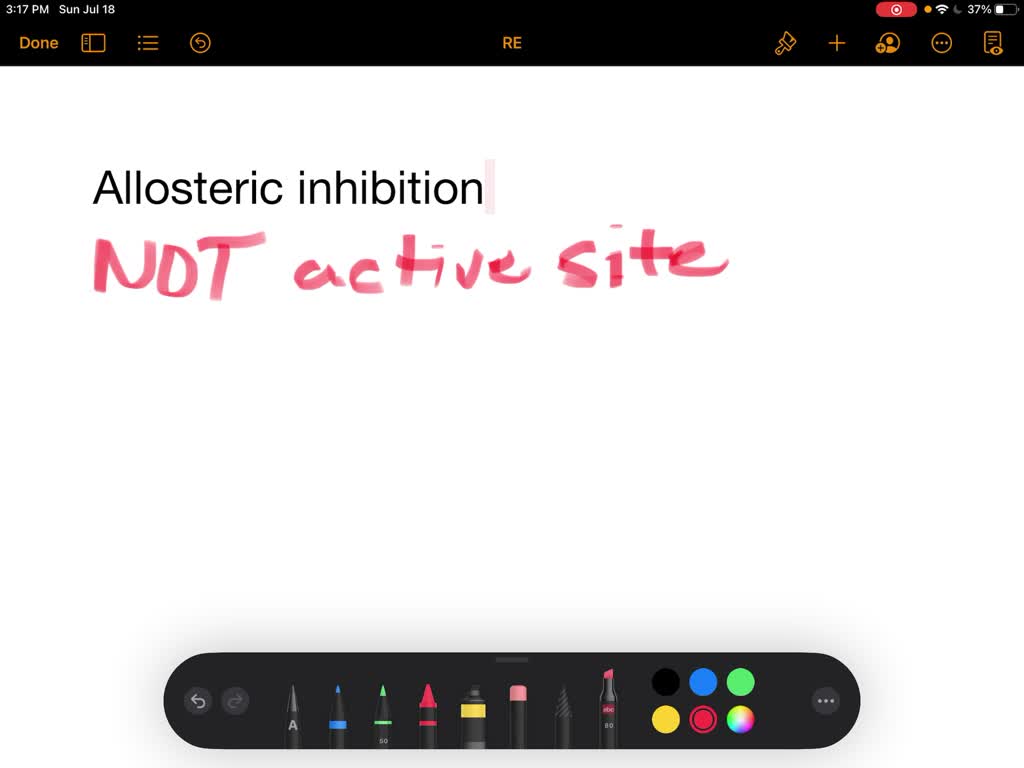 Source: numerade.com
Source: numerade.com
The following statements are true for feedback allosteric inhibition in multienzyme system: Some examples of irreversible inhibitors include nerve gas,. An allosteric inhibitor does which of the following?
 Source: studocu.com
Source: studocu.com
Binds to the active site and blocks it from binding substrate. The existence of allosteric sites on receptor molecules has expanded potential drug mechanisms. This causes the substrate to be unable to bind to the active site.
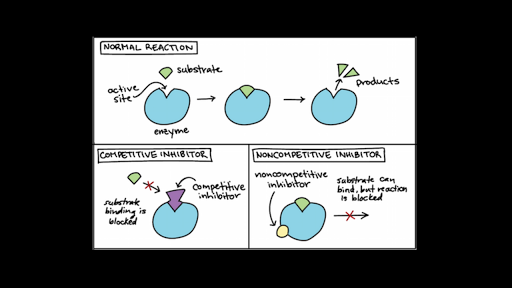
The active site changes shape when an inhibitor binds to an allosteric site. When an allosteric inhibitor binds to an enzyme, all active sites on the protein subunits are changed slightly so that they work less well. The inhibition of deoxythymidilate formation and subsequent blockage of cell division is due to a.

An allosteric inhibitor does which of the following? Amp diminishes and citrate enhances the inhibitory effect of atp. Kinetics of an allosteric enzyme.
 Source: chegg.com
Source: chegg.com
Binds to an enzym… 00:49. An allosteric inhibitor bound to one subunit alters substrate binding to other subunits; Amp diminishes and citrate enhances the inhibitory effect of atp.
 Source: numerade.com
Source: numerade.com
Allosteric enzymes have active and inactive shapes differing in 3d structure. •it is for modifiers to bind. Allosteric enzymes often have multiple inhibitor or activator binding sites involved in switching between active and inactive shapes.
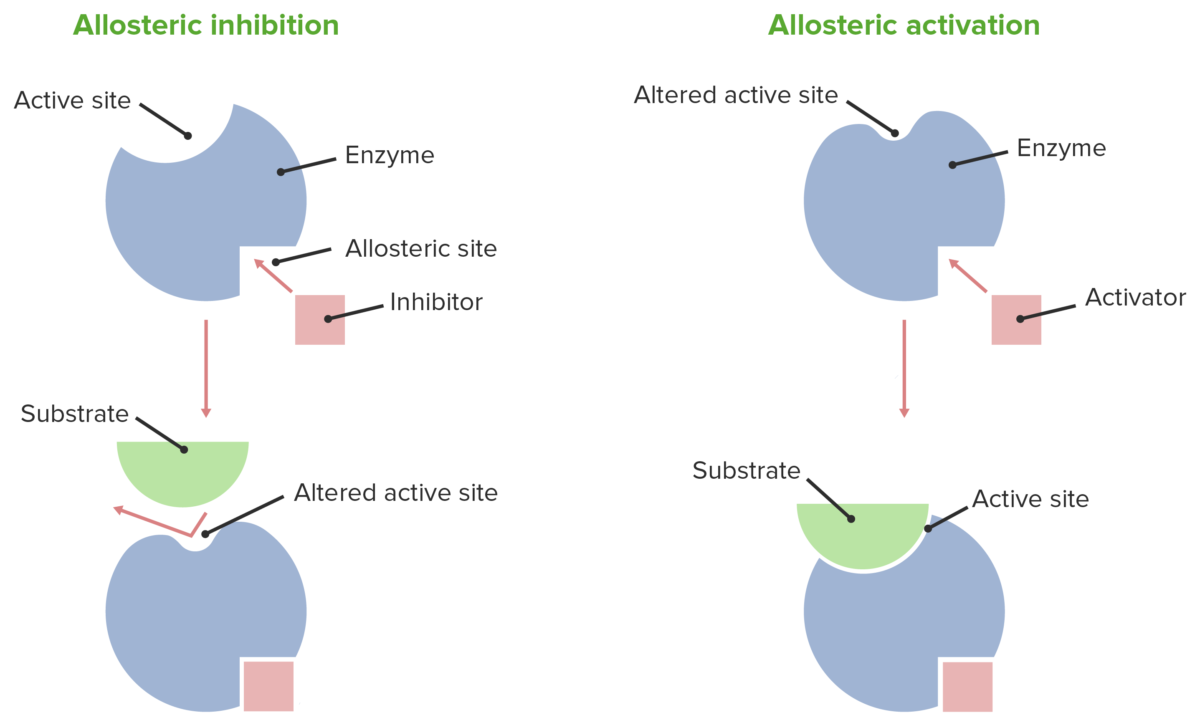 Source: lecturio.com
Source: lecturio.com
Amp diminishes and citrate enhances the inhibitory effect of atp. The active site changes shape when an inhibitor binds to an allosteric site. Kinetics of an allosteric enzyme.
![Solved] Question 12 In Which Of The Following Ways Does An Allosteric Inhibitor Affect The Rate Of A Reaction? | Course Hero](https://www.coursehero.com/qa/attachment/13143840/ “Solved] Question 12 In Which Of The Following Ways Does An Allosteric Inhibitor Affect The Rate Of A Reaction? | Course Hero”) Source: coursehero.com
This causes a conformational change in the active site for the second molecule, preventing binding. Allosteric enzymes are enzymes that have an additional binding site for effector molecules other than the active site. Which is an example of irreversible inhibitor?
 Source: numerade.com
Source: numerade.com
An allosteric inhibitor does which of the following? An allosteric inhibitor does which of the following? The allosteric activator binds to an enzyme at a site other than the active site.
 Source: khanacademy.org
Source: khanacademy.org
Which is an example of irreversible inhibitor? Allosteric enzymes typically have multiple active sites located on different protein subunits. Binds to the active site and blocks it from binding substrate.
 Source: biologydictionary.net
Source: biologydictionary.net
Which of the following is the feedback inhibition? In allosteric regulation, effector (inhibitor or activator) binds to a site other than the active site to bring about conformational changes and thereby affecting the activity of the enzyme. An enzyme inhibitor is a substance that binds with the enzyme and brings about a decrease in the catalytic activity of that enzyme.
 Source: study.com
Source: study.com
The active site changes shape when an inhibitor binds to an allosteric site. O after dissociation of allosteric inhibitor from enzyme, substrate can bind to enzyme at active site o binding of allosteric inhibitor to the active site on enzyme o binding of substrate to active site. Binds to an enzym… 0:00.
 Source: en.wikipedia.org
Source: en.wikipedia.org
• first enzyme of the sequence is regulatory allosteric enzyme. The substrate concentration is equal to that of a competitive inhibitor b. Allosteric enzymes have active and inactive shapes differing in 3d structure.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
An allosteric inhibitor bound to one subunit alters substrate binding to other subunits; O after dissociation of allosteric inhibitor from enzyme, substrate can bind to enzyme at active site o binding of allosteric inhibitor to the active site on enzyme o binding of substrate to active site. This can be classified into the following types as.
Also Read :





