Among the intermolecular forces, which forces are typically the weakest? Y's molecules experience stronger london dispersion forces than x's molecules.
Among The Intermolecular Forces Which Forces Are Typically The Weakest. The london dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. Ionic bondthe weakest of the intramolecular bonds or chemical bonds is the ionic bond. The bond strengths described range from strongest to weakest (the latter 3 are examples of van der waals forces). The london dispersion force is the weakest intermolecular force.
 10.1 Intermolecular Forces – Chemistry From opentextbc.ca
10.1 Intermolecular Forces – Chemistry From opentextbc.ca
Related Post 10.1 Intermolecular Forces – Chemistry :
Because it involves highly electronegative (tendency of an atom to attract electrons) e.g. These forces are thought to exist between all types of atoms and molecules when they are sufficiently near each other. Among the intermolecular forces, which forces are typically the weakest? Among the intermolecular forces, which forces are typically the weakest?
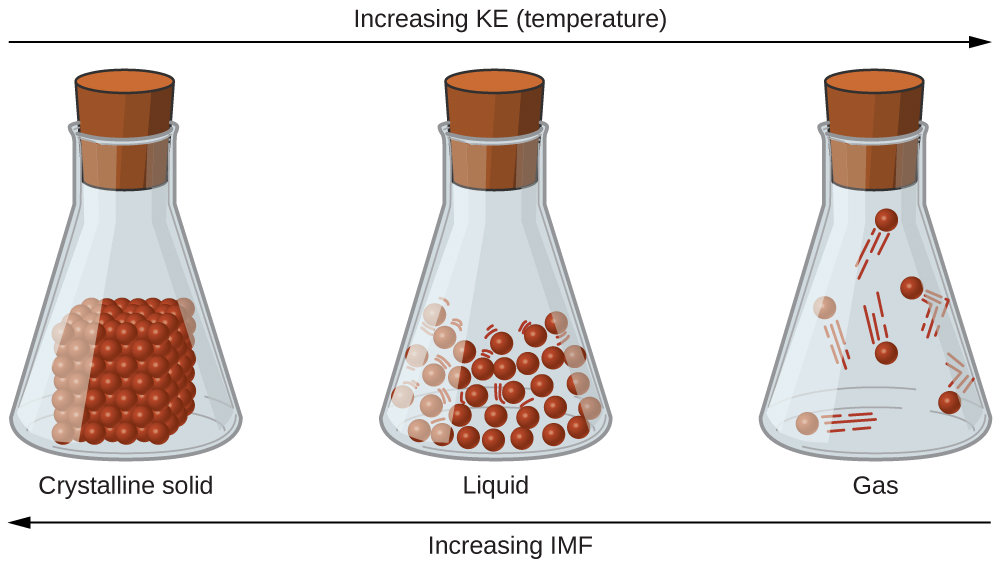
There are three different types of intermolecular forces in terms of strength.
London dispersion forces, dipole interaction forces, hydrogen bonding is the list among the following choices given in the question that correctly orders intermolecular forces from weakest to strongest. London dispersion forces in the molecule on the left, areas that have a partial negative charge are pink and areas that have a partial positive charge are blue. The bond strengths described range from strongest to weakest (the latter 3 are examples of van der waals forces). Ionic bondthe weakest of the intramolecular bonds or chemical bonds is the ionic bond. Experts are tested by chegg as specialists in their subject area. Among the intermolecular forces, which forces are typically the weakest?
 Source: study.com
Source: study.com
Because it involves highly electronegative (tendency of an atom to attract electrons) e.g. London dispersion forces which statement best describes the effect of low lonization energies and low electronegativities on metallic bonding? Next the polar covalent bond and the strongest the non polar covalent bond.
 Source: chegg.com
Source: chegg.com
London dispersion forces in the molecule on the left, areas that have a partial negative charge are pink and areas that have a partial positive charge are blue. Ionic bondthe weakest of the intramolecular bonds or chemical bonds is the ionic bond. These intermolecular forces bind molecules to molecules.

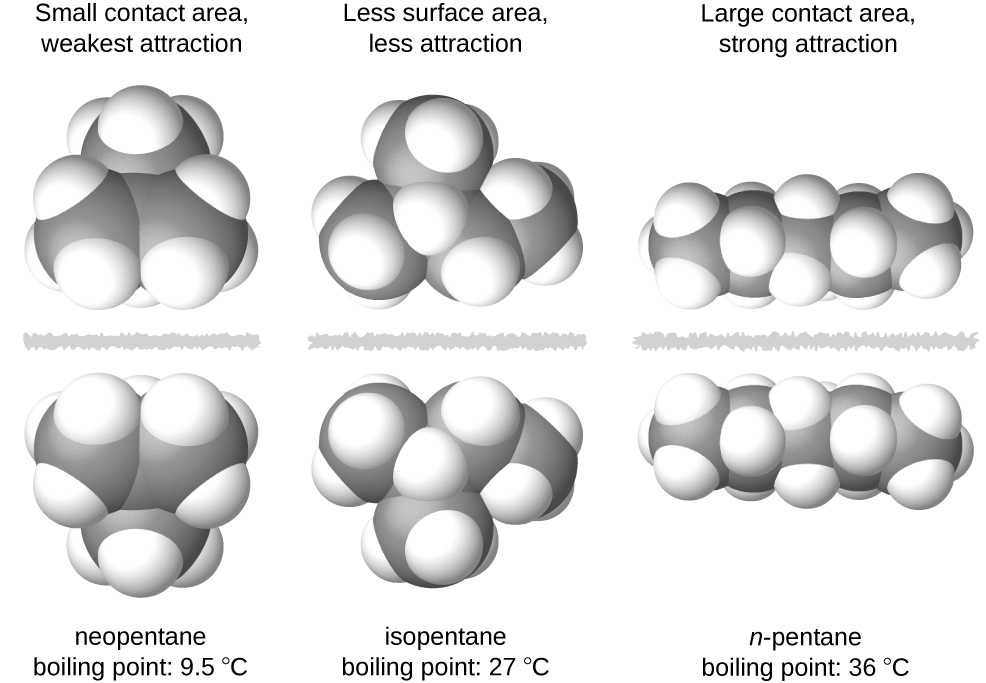
Neopentane molecules are the most compact of the three, offering the least available surface area for intermolecular contact and, hence, the weakest dispersion forces. What are the strongest and weakest forces of attraction between molecules? The bond strengths described range from strongest to weakest (the latter 3 are examples of van der waals forces).
 Source: chem.libretexts.org
Source: chem.libretexts.org
- option b is answer the london dispersion force or the vander wall forces are the weakest intermolecular force because these are the temporary attractive forces that results when the electrons in two ad. London dispersion forces, dipole interaction forces, hydrogen bonding is the list among the following choices given in the question that correctly orders intermolecular forces from weakest to strongest. Among the intermolecular forces, which forces are typically the weakest?
![Solved] 1. Which Among The Intermolecular Forces Is The Weakest? A. Hydrogen Bond B. Ion-Dipole Forces C. Dispersion Forces D. Dipole-Dipole Forces… | Course Hero](https://www.coursehero.com/qa/attachment/18176805/ “Solved] 1. Which Among The Intermolecular Forces Is The Weakest? A. Hydrogen Bond B. Ion-Dipole Forces C. Dispersion Forces D. Dipole-Dipole Forces… | Course Hero”) Source: coursehero.com
What is the weakest force in chemistry? Neopentane molecules are the most compact of the three, offering the least available surface area for intermolecular contact and, hence, the weakest dispersion forces. These are the weakest of the intermolecular forces and exist between all types of molecules, whether ionic or covalent—polar or nonpolar.
 Source: opentextbc.ca
Source: opentextbc.ca
We review their content and use your feedback to keep the quality high. The london dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. These are the weakest of the intermolecular forces and exist between all types of molecules, whether ionic or covalent—polar or nonpolar.

Among the intermolecular forces, which forces are typically the weakest? Next the polar covalent bond and the strongest the non polar covalent bond. We review their content and use your feedback to keep the quality high.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
London dispersion forces tend to be the weakest intermolecular forces. London dispersion forces tend to be the weakest intermolecular forces. What is the weakest force in chemistry?
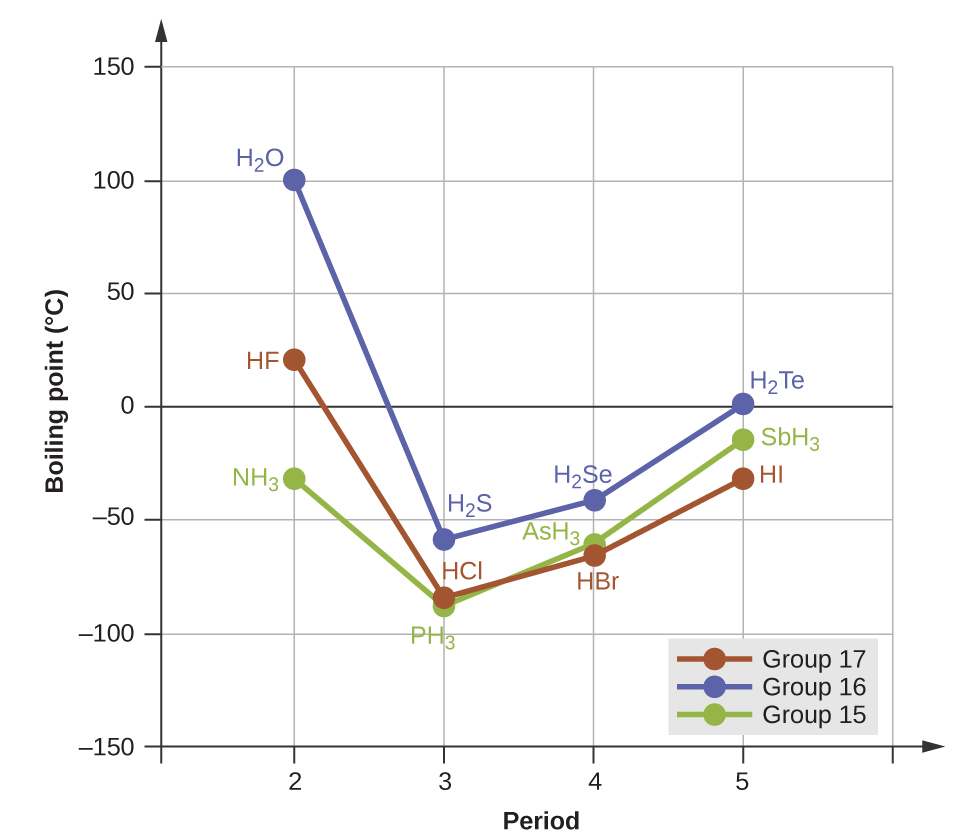 Source: opentextbc.ca
Source: opentextbc.ca
Added 8/26/2018 1:27:49 pm this answer has been confirmed as correct and helpful. What are the strongest and weakest forces of attraction between molecules? Ionic bondthe weakest of the intramolecular bonds or chemical bonds is the ionic bond.
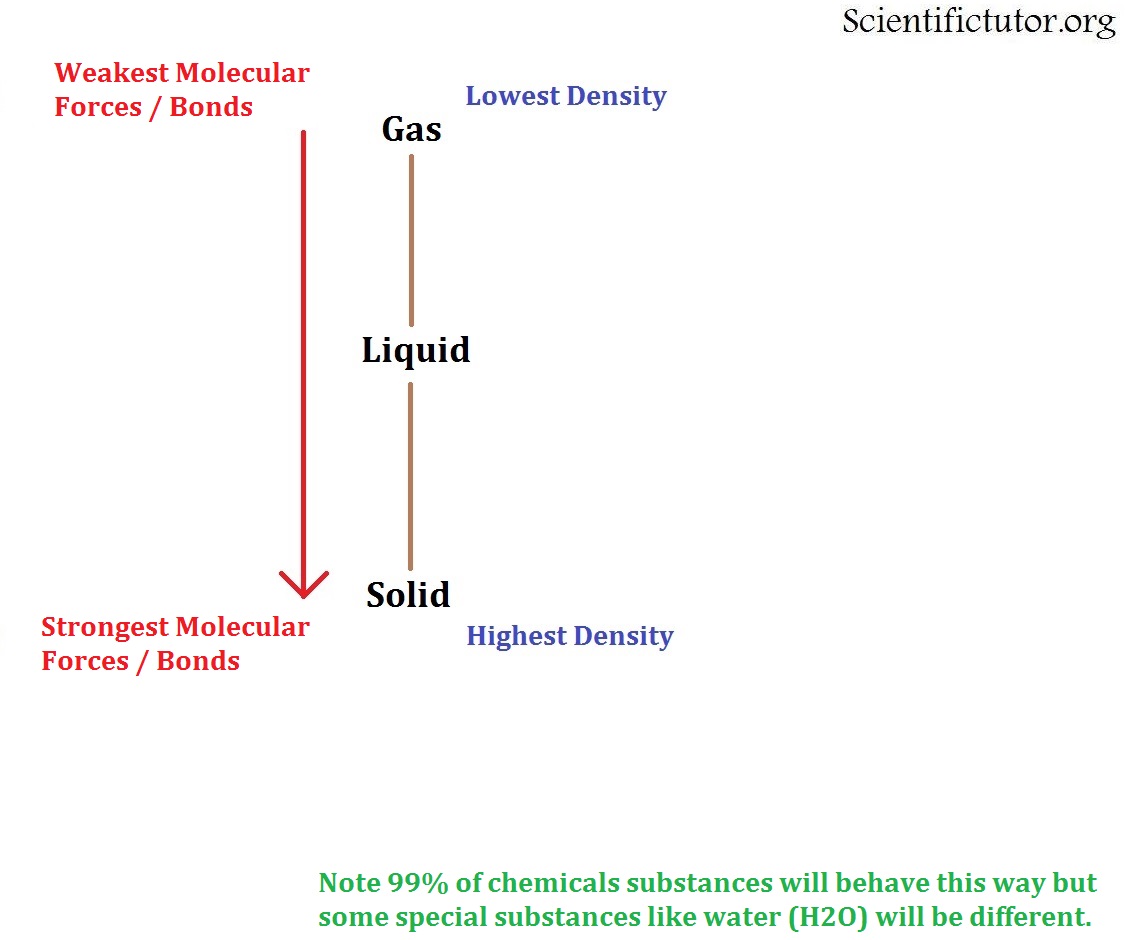 Source: scientifictutor.org
Source: scientifictutor.org
Among the intermolecular forces, which forces are typically the weakest? Neopentane molecules are the most compact of the three, offering the least available surface area for intermolecular contact and, hence, the weakest dispersion forces. Among the intermolecular forces, which forces are typically the weakest?
 Source: opentextbc.ca
Source: opentextbc.ca
- option b is answer the london dispersion force or the vander wall forces are the weakest intermolecular force because these are the temporary attractive forces that results when the electrons in two ad. London dispersion forces tend to be the weakest intermolecular forces. What are the strongest and weakest forces of attraction between molecules?
![Solved] 1. Which Among The Intermolecular Forces Is The Weakest? A. Hydrogen Bond B. Ion-Dipole Forces C. Dispersion Forces D. Dipole-Dipole Forces… | Course Hero](https://www.coursehero.com/qa/attachment/18176816/ “Solved] 1. Which Among The Intermolecular Forces Is The Weakest? A. Hydrogen Bond B. Ion-Dipole Forces C. Dispersion Forces D. Dipole-Dipole Forces… | Course Hero”) Source: coursehero.com
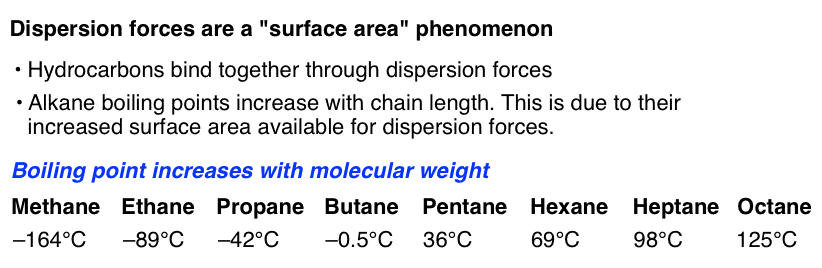
Compounds ii and iii only exhibit intermolecular london dispersion forces, so they would be the two lowest boiling compounds (weakest intermolecular forces). Because compound iii has more branching, these london dispersion forces would be weaker, resulting in a lower boiling point than compound ii. The london dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles.
 Source: brainly.com
Source: brainly.com
These are the weakest of the intermolecular forces and exist between all types of molecules, whether ionic or covalent—polar or nonpolar. What is the weakest force in chemistry? Because it involves highly electronegative (tendency of an atom to attract electrons) e.g.
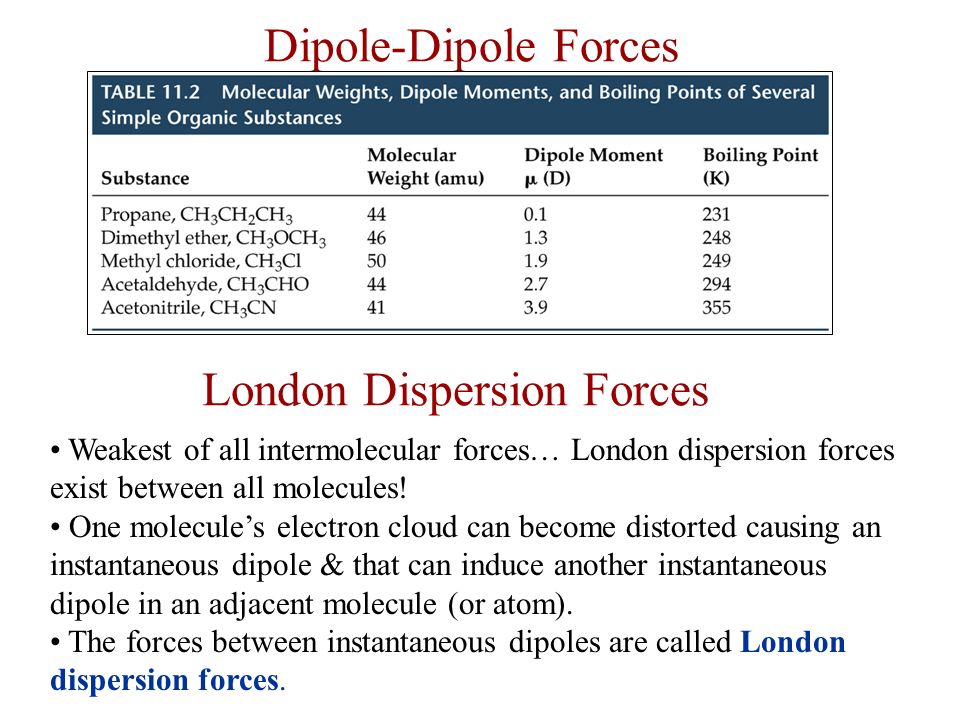 Source: slideplayer.com
Source: slideplayer.com
Because it involves highly electronegative (tendency of an atom to attract electrons) e.g. The correct option among all the options that are given in the question is the first option or option a. Hence, london dispersion forces are the weakest.

Among the intermolecular forces, which forces are typically the weakest? These are the weakest of the intermolecular forces and exist between all types of molecules, whether ionic or covalent—polar or nonpolar. And hydrogen has only one electron, therefore is less negative (almost positive in a sense).
 Source: slideplayer.com
Source: slideplayer.com
London dispersion forces in the molecule on the left, areas that have a partial negative charge are pink and areas that have a partial positive charge are blue. Such intermolecular forces are called van der waals forces and they have nothing to do with the valence electrons. Ionic bondthe weakest of the intramolecular bonds or chemical bonds is the ionic bond.
 Source: doubtnut.com
Source: doubtnut.com
Among the intermolecular forces, which forces are typically the weakest? There are even weaker intermolecular “bonds” or more correctly forces. Hence, london dispersion forces are the weakest.
 Source: khanacademy.org
Source: khanacademy.org
The london dispersion force is the weakest intermolecular force. Among the intermolecular forces, which forces are typically the weakest? The london dispersion force is the weakest intermolecular force.
 Source: chem.libretexts.org
Source: chem.libretexts.org
These intermolecular forces bind molecules to molecules. Hence, london dispersion forces are the weakest. The bond strengths described range from strongest to weakest (the latter 3 are examples of van der waals forces).
 Source: khanacademy.org
Source: khanacademy.org
Hence, london dispersion forces are the weakest. What is the weakest force in chemistry? Compounds ii and iii only exhibit intermolecular london dispersion forces, so they would be the two lowest boiling compounds (weakest intermolecular forces).
Also Read :