Mesomers are a type of compounds in which net rotation of plane polarised light is zero. Investigations of isomeric tartaric acid salts, carried out by louis pasteur in the mid 19th century, were instrumental in elucidating some of the subtleties of stereochemistry.
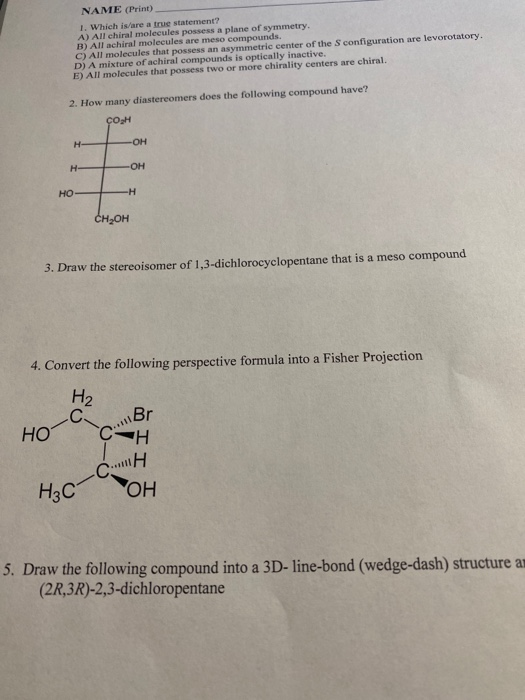
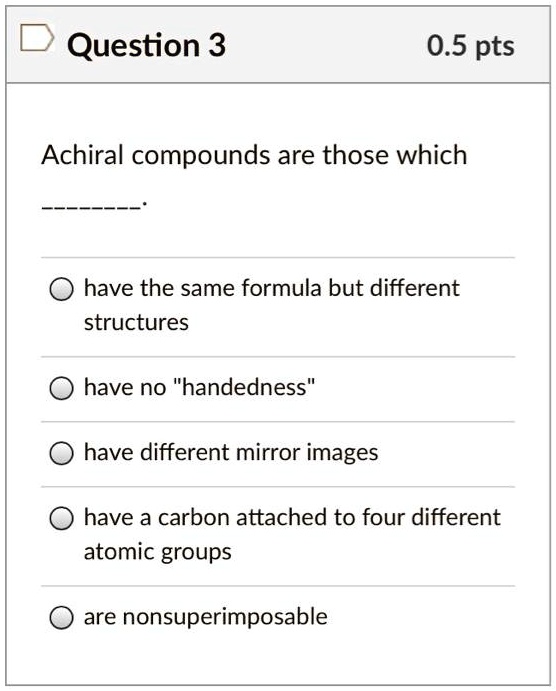
Achiral Compounds Are Those Which. Investigations of isomeric tartaric acid salts, carried out by louis pasteur in the mid 19th century, were instrumental in elucidating some of the subtleties of stereochemistry. The main difference between achiral and meso is that achiral compounds have no chiral centers whereas meso compounds are intermediate to chiral and achiral compounds. Chiralpak ie, eluent ethanol/dichloromethane 30/70, v/v; Hydrogen is attached to them.
 Difference Between Achiral And Meso | Definition, Structure, Examples, How To Identify From pediaa.com
Difference Between Achiral And Meso | Definition, Structure, Examples, How To Identify From pediaa.com
Related Post Difference Between Achiral And Meso | Definition, Structure, Examples, How To Identify :
Compound c has a plane of symmetry, so it is clearly achiral. So , the compounds which do not show such type of property , does not rotate plane polarised light are optically inactive and are called achiral. Chiral compound do not superimpose. Achiral compounds and meso compounds are related to each other in some properties but have some different properties as well.
Chiralpak ie, eluent ethanol/dichloromethane 30/70, v/v;
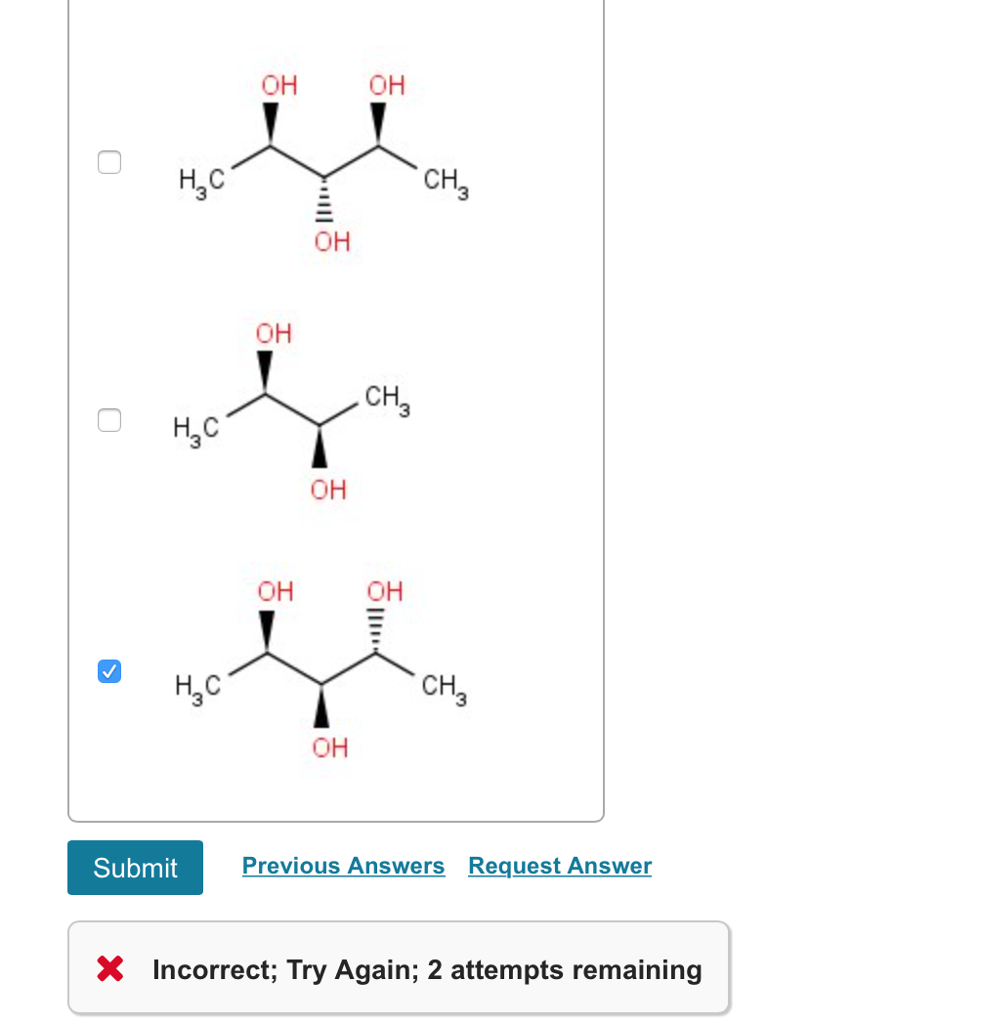
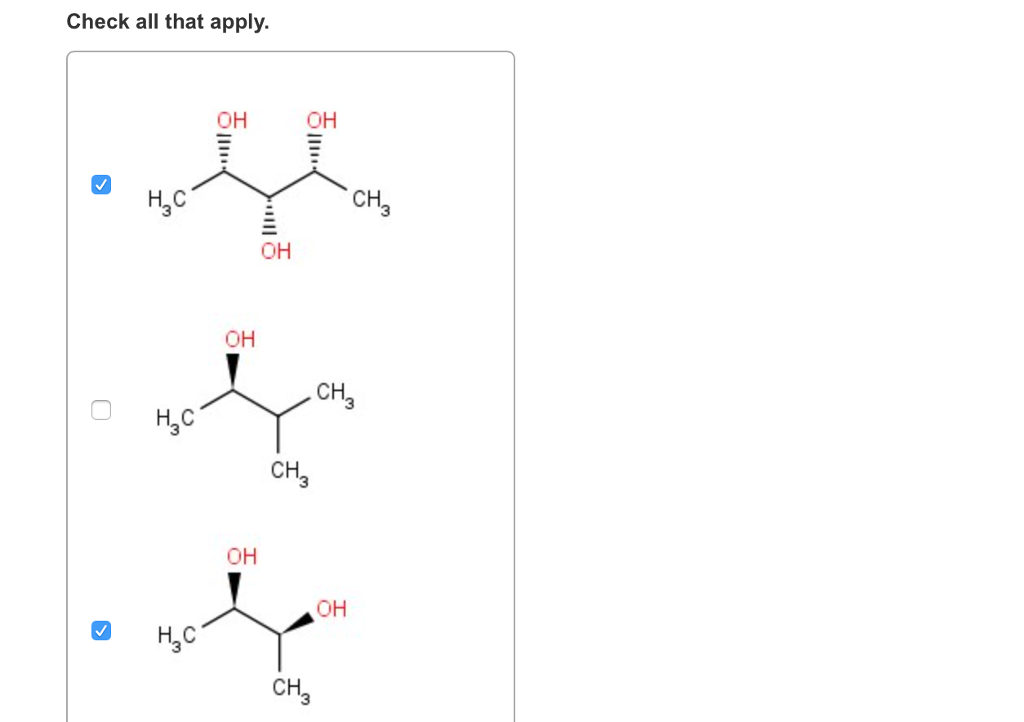
(each of your choices can be achiral in any conformation, not just the ones shown.) check all that apply. Mesomers are a type of compounds in which net rotation of plane polarised light is zero. A meso compound is an achiral compound that has chiral centers. So we can�t really say those air cairo centers the nitrogen, since it only has three. Which is the canonical example of an object with this property. This geometric property is called chirality.
 Source: chegg.com
Source: chegg.com
Some molecules are achiral even though they contain chirality centers. Choose the achiral structures choose all the achiral compounds among the ones shown. So , the compounds which do not show such type of property , does not rotate plane polarised light are optically inactive and are called achiral.
 Source: study.com
Source: study.com
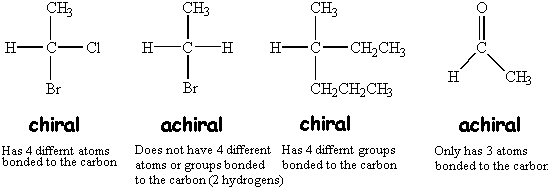
A meso compound is an achiral compound that has chiral centers. Chiral surfaces are critical components of enantioselective heterogeneous processes such as those used to prepare enantiomerically pure pharmaceuticals. A chiral compound is a molecule having a carbon atom attached to four different substituents.
 Source: chegg.com
Source: chegg.com
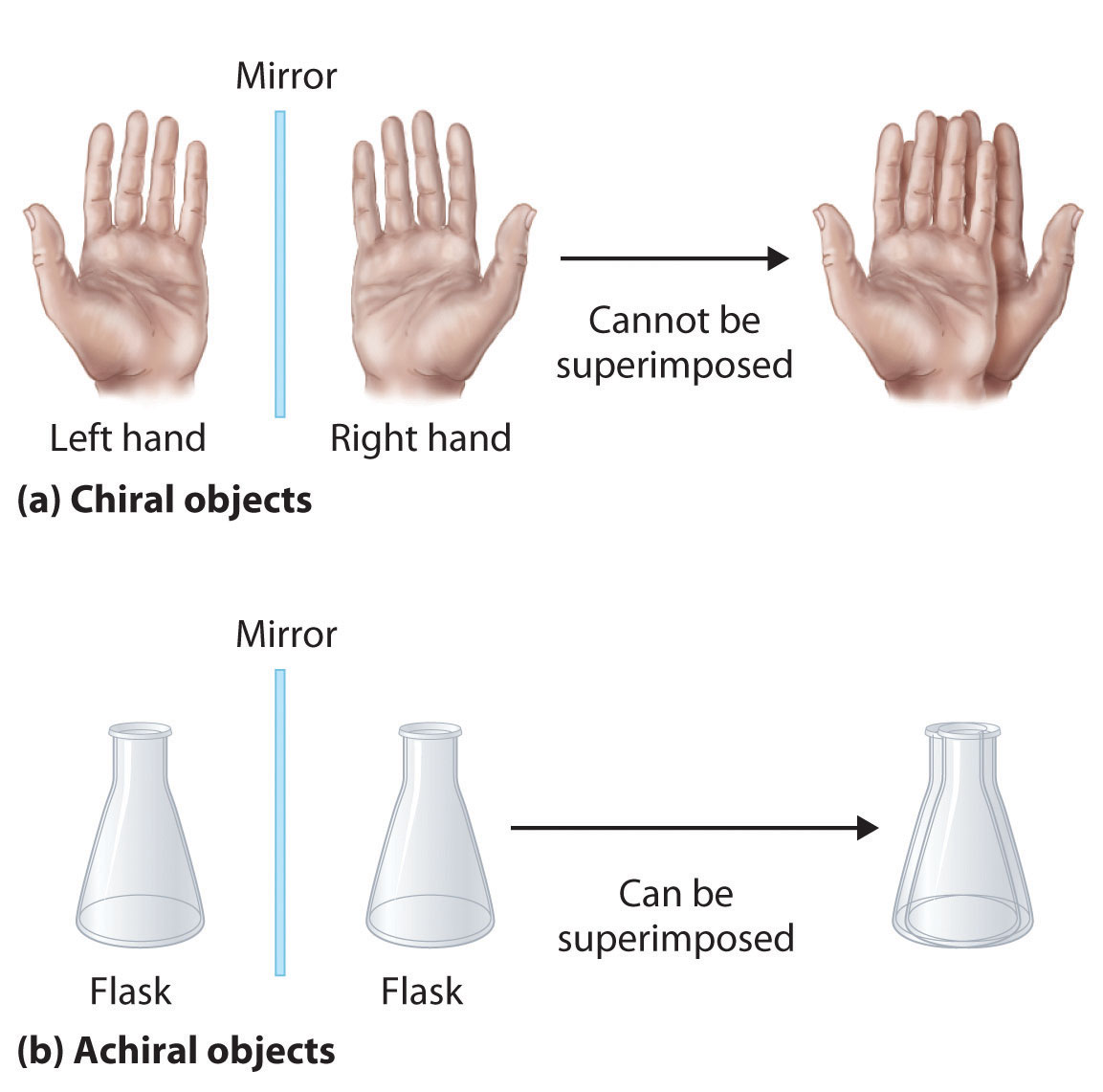
Thus, 1 is optically inactive. Optically active compounds are those which can rotate the plane of plane polarised light, and those which not are optically inactive compounds. The terms are derived from ancient greek χείρ (cheir), meaning hand;
 Source: pediaa.com
Source: pediaa.com
Meso compounds are achiral (optically inactive) diastereomers of chiral stereoisomers. The key difference between the terms achiral and meso is that achiral compounds have no chiral centers whereas meso compounds have multiple chiral centers. Investigations of isomeric tartaric acid salts, carried out by louis pasteur in the mid 19th century, were instrumental in elucidating some of the subtleties of stereochemistry.
 Source: chegg.com
Source: chegg.com
Chiral compound do not superimpose. All pure achiral compounds are optically inactive. Pyridine, deap, amino, nitro and cyano.
 Source: sciencedirect.com
Source: sciencedirect.com
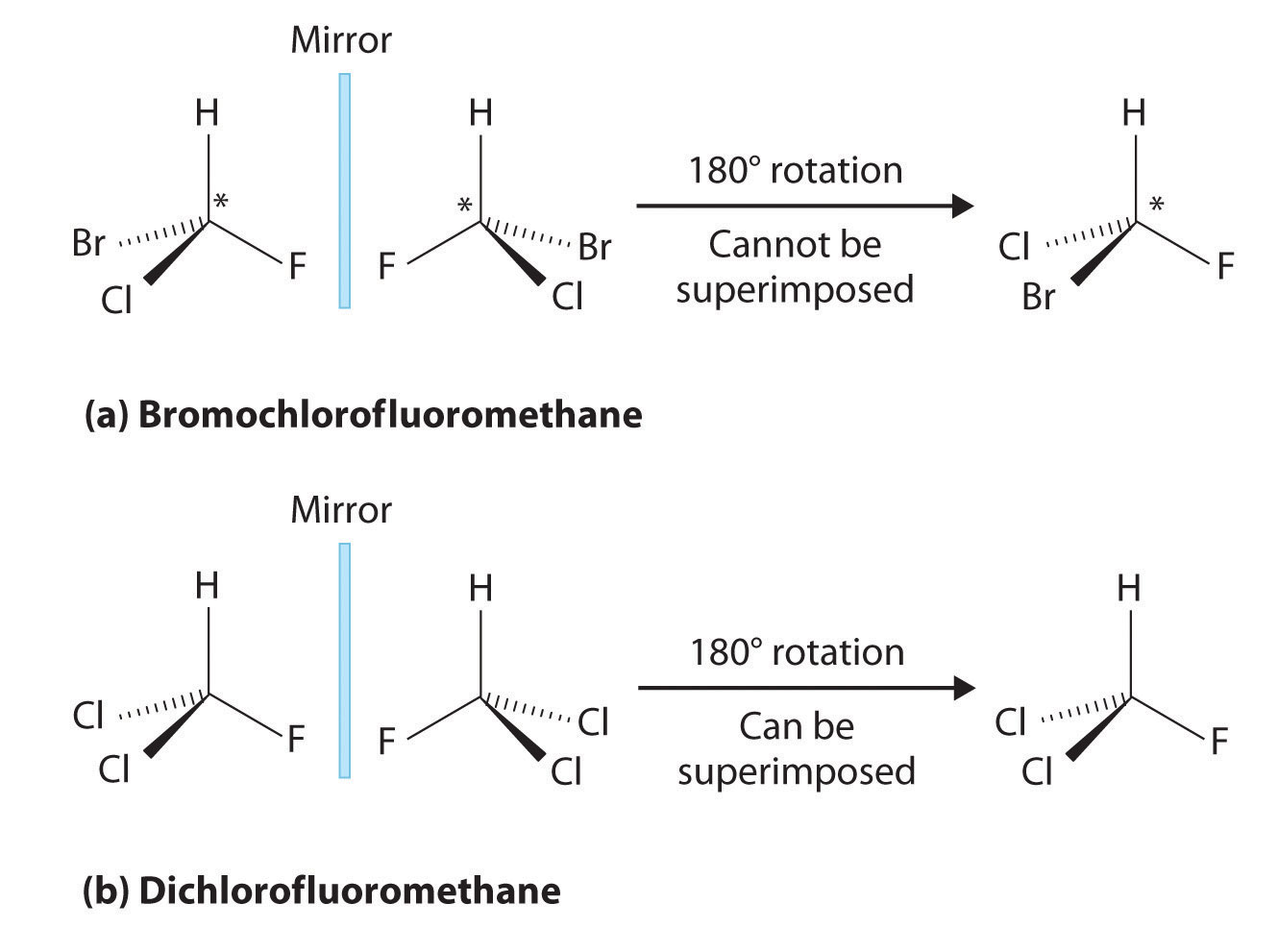
Achiral (not chiral) objects are those objects that are identical to their mirror image. Tartaric acid [ho,cch(oh)ch(oh)co,h] was an important compound in the history of stereochemistry. A meso compound is an achiral compound that has chiral centers.
 Source: chegg.com
Source: chegg.com
I.e to be simple , mesomers are type of organic compounds where two chiral carbons are present and those two are similar , so net rotation is zero. In summary, an achiral compound is the opposite of a chiral compound. So , the compounds which do not show such type of property , does not rotate plane polarised light are optically inactive and are called achiral.
 Source: chegg.com
Source: chegg.com
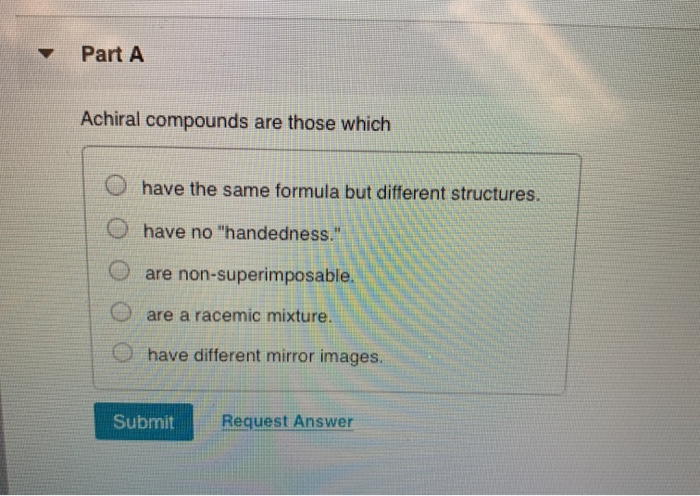
Part a achiral compounds are those which have the same formula but different structures. The analytical and preparative resolutions of the porphyrin cage compounds were performed by chiral hplc (compound 1: Meso compound an achiral compound possessing two or more chiral centers that also has chiral isomers
 Source: numerade.com
Source: numerade.com
- stereoisomers that are nonsuperimposable mirror images of each other are known as _____. Mesomers are a type of compounds in which net rotation of plane polarised light is zero. (each of your choices can be achiral in any conformation, not just the ones shown.) check all that apply.
 Source: socratic.org
Source: socratic.org
Chiral compound do not superimpose. See the answer see the answer see the answer done loading. All pure achiral compounds are optically inactive.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Mesomers are a type of compounds in which net rotation of plane polarised light is zero. This problem has been solved! Meso compound an achiral compound possessing two or more chiral centers that also has chiral isomers
 Source: chegg.com
Source: chegg.com
However, as you suggest, chair flip of b gives the mirror image of the original drawing, which means the original and mirror image are equivalent, assuming. Mesomers are a type of compounds in which net rotation of plane polarised light is zero. A meso compound is an achiral compound that has chiral centers.
 Source: sciencedirect.com
Source: sciencedirect.com
Chiral surfaces are critical components of enantioselective heterogeneous processes such as those used to prepare enantiomerically pure pharmaceuticals. Hydrogen is attached to them. See the answer see the answer see the answer done loading.
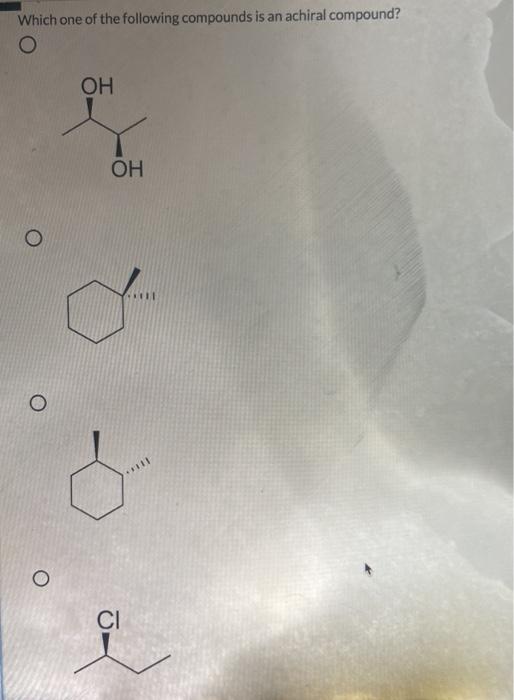
So we can�t really say those air cairo centers the nitrogen, since it only has three. Part a achiral compounds are those which have the same formula but different structures. 12) stereoisomers that are nonsuperimposable mirror images of each other are known as _____.
 Source: chegg.com
Source: chegg.com
A meso compound is an achiral compound that has chiral centers. Mesomers are a type of compounds in which net rotation of plane polarised light is zero. In summary, an achiral compound is the opposite of a chiral compound.
 Source: study.com
Source: study.com
The main difference between achiral and meso is that achiral compounds have no chiral centers whereas meso compounds are intermediate to chiral and achiral compounds. A chiral compound is a molecule having a carbon atom attached to four different substituents. In summary, an achiral compound is the opposite of a chiral compound.
 Source: youtube.com
Source: youtube.com
And then all the carbons out here. Thus, 1 is optically inactive. Since our compounds are primarily basic, we chose to prescreen using the following stationary phases:
 Source: chegg.com
Source: chegg.com
E) have a carbon attached to four different atoms. So , the compounds which do not show such type of property , does not rotate plane polarised light are optically inactive and are called achiral. Compound c has a plane of symmetry, so it is clearly achiral.
 Source: socratic.org
Source: socratic.org
Compound c has a plane of symmetry, so it is clearly achiral. The key difference between the terms achiral and meso is that achiral compounds have no chiral centers whereas meso compounds have multiple chiral centers. The analytical and preparative resolutions of the porphyrin cage compounds were performed by chiral hplc (compound 1:
 Source: chegg.com
Source: chegg.com
Thus, 1 is optically inactive. So we can�t really say those air cairo centers the nitrogen, since it only has three. Achiral compounds and meso compounds are related to each other in some properties but have some different properties as well.
Also Read :





